Office of Generic Drugs (OGD) Annual Report for 2016
Ensuring Safe, Effective, and Affordable Medicines for the American Public
Approved 630 abbreviated new drug applications (ANDAs) and tentatively approved 183—the highest number of generic drug approvals and tentative approvals in the history of the generic drug program.
Met required GDUFA review timeframes.
Published more than 200 product-specific guidances related to developing generic drugs, for a total of more than 1,500 posted on FDA’s website.
Issued first approvals for generic versions of commonly used drugs including Benicar, Viagra, Crestor, and Tamiflu.
Awarded funding to 16 new external researchers to conduct regulatory science activities that will complement FDA’s research efforts.
Supported 87 ongoing external research collaborations.
Verified validity of FDA’s bioequivalence standards for certain drugs through scientific studies, demonstrating the proven efficacy and safety of generic drugs.

It is exciting to see the number of approvals and tentative approvals continuing to rise, but our main focus is always to ensure the safety, effectiveness, and quality of FDA-approved drugs. FDA-approved generic drugs account for 89 percent of prescriptions dispensed in the United States.2 They must meet high standards to ensure that they can be substituted for the brand-name drug.
This year, we approved 73 first generic drugs, which introduce an alternative for a brand-name product where there was previously none. First generics, in particular, help reduce the cost of high-priced brand-name drugs. Multiple generic versions of brand-name drugs are also important contributors to price competition, leading to more affordable drugs. Use of generic drugs saved the U.S. health system almost $1.5 trillion in the past 10 years3, leading to cost savings for consumers.
The Generic Drug User Fee Amendments (GDUFA) of 2012 authorized additional funds for FDA to review generic drug applications, inspect facilities, and perform other regulatory actions. GDUFA specified that by 2017, FDA would take action on 90 percent of the applications that were pending prior to the start of GDUFA. This year we reached that milestone—more than a year ahead of schedule.
In 2016, we approved 526 prior approval supplements (PASs). We also communicated with industry through more than 4,800 information requests, more than 1,800 controlled correspondences, and more than 1,800 complete response letters detailing comments and questions that need to be addressed by the applicant before FDA can continue with review of the application.
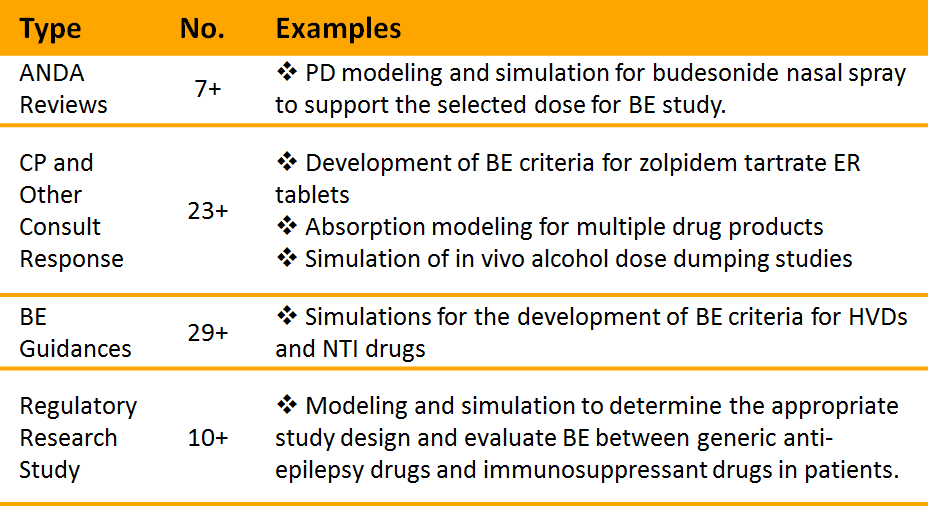
Input from industry and other stakeholders helps FDA develop an annual list of FDA’s regulatory science priorities. Based on the identified priorities, FDA researches scientific methods and clinically relevant bioequivalence testing, which requires thorough understanding of the brand-name drug. OGD – with GDUFA funding – is able to conduct and disseminate the necessary research while protecting the proprietary rights of the brand-name drug manufacturer. The results of the regulatory science work done with GDUFA funding helps industry make generic versions of brand-name medications by building research and generic drug development capabilities necessary for the development of a generic drug product.
We have also begun leveraging international generic drug activities to better understand drivers of the global drug market, which is critical to ensuring consistent quality in generic drugs sold in the United States. Nearly 80 percent of generic drugs have a global aspect to their development or production. We began to engage with the FDA’s Office of International Programs and CDER’s Office of Strategic Planning, to better work with FDA international offices, regional regulators, and foreign industry in India, China, and Latin America. We developed programs for working with the International Conference on Harmonization on regulation, manufacturing, and inspection for several aspects of generic drug application and review. We are exploring how to best work with other international organizations, such as the International Generic Drug Regulators Programme. We anticipate that these collaborations will ultimately lead to improved ANDAs and a greater consistency in the quality of generic drug products developed internationally.
We look forward to working with industry, the research community, lawmakers, patients, and other stakeholders to promote the public health and reduce the cost of medical therapy by increasing access to high-quality, affordable generic drugs.
Kathleen Uhl, MD
Director, Office of Generic Drugs
_____________________________________________
1Tentative approvals are granted to applications ready for approval from a scientific perspective, but cannot be fully approved due to patents or exclusivities on the brand-name drug.
2 IMS Institute for Healthcare Informatics
3 Generic Drug Savings in the U.S. Seventh Annual Edition: 2015, available at http://www.gphaonline.org/media/wysiwyg/PDF/GPhA_Savings_Report_2015.pdf
Center for Biologics Evaluation and Research
Center for Devices and Radiological Health
Center for Drug Evaluation and Research
Office of Communications
Office of Compliance
Office of Management
Office of Medical Policy
Office of New Drugs
Office of Pharmaceutical Quality
Office of Strategic Programs
Office of Surveillance and Epidemiology
Office of Translational Sciences
- Office of Chief Counsel
- Office of the Commissioner
- Office of Regulatory Affairs
OGD is comprised of an immediate office and four subordinate offices. OGD hired approximately 125 new employees in 2016, bringing the office to about 450 employees.
Immediate Office (IO)
Clinical Safety Surveillance Staff (CSSS)
Obtains and coordinates information regarding the safety and surveillance of generic drug products.
Serves as OGD's liaison to CDER’s Office of Surveillance and Epidemiology (OSE) and other drug surveillance units within CDER.
Interacts with external stakeholders such as physicians, pharmacists, patients, and patient advocacy groups to investigate reports of adverse events or therapeutic inequivalence of generic drugs.
Communications Staff (CS)
- Oversees and coordinates all communications that originate from OGD.
- Liaises with CDER’s Office of Communications and FDA’s Office of Media Affairs on all generic drug topics.
- Plays a strategic role in communicating accurate information on the approval and surveillance of generic drugs.
Generic Regulatory Affairs Team (GReAT)
- Provides oversight, outreach, strategic liaison, and integration of cross-OGD and cross-Center generic drug regulatory programs and initiatives.
Program Management and Analysis Staff (PMAS)
- Provides leadership, guidance, and support services to OGD on all aspects of budget, contracts, facilities management, human resources, personnel operations services, scientific fellowships and recruitment activities.
OGD’s global affairs function coordinates OGD’s proactive international stakeholder engagement.
Office of Bioequivalence (OB)
The divisions within the OB evaluate formulations for quantitative and qualitative (Q1/Q2) equivalence and review bioequivalence (BE) studies, including those with pharmacokinetic and pharmacodynamic endpoints. They collaborate with other CDER and OGD Offices to consider newer methodologies for demonstrating BE in complex dosage forms. They also provide CSSS with investigation of products that have been identified as having potential safety or therapeutic inequivalence issues.
The REMS Team works to identify generic drug applications affected by a Risk Evaluation Mitigation Strategies (REMS). REMS are risk management plans that use risk minimization strategies beyond professional labeling to ensure that the benefits of certain prescription drugs outweigh their risks. These include ANDAs that must have a REMS in order to be approved, generic drugs with REMS that impact labeling, and post-approval supplements of ANDAs that require a modification to the REMS.
The OB’s Division of Clinical Review evaluates BE studies with clinical endpoints, skin adhesion and irritation/sensitization studies for transdermal delivery systems, REMS protocols, and BE studies for investigational new drugs (Bio-INDs). The division reviews suitability petitions, citizen petitions, relisting/delisting reviews, and controlled correspondences related to clinical issues. It also responds to consult requests related to clinical or pharmacology/toxicology issues, and collaborates with the Office of Research and Standards on BE recommendations and with CSSS on post-marketing surveillance investigations.
Office of Generic Drug Policy (OGDP)
Identifies legally defensible regulatory options for OGD that actively promote OGD’s mission of ensuring safe, effective, and affordable drugs for the American public
Ensures consistency in generic drug regulatory review standards and processes through development and implementation of policy documents
Advises CDER and OGD on Hatch-Waxman patent and exclusivity matters; and statutory and regulatory issues directly related to ANDAs
Maintains the Approved Drug Products with Therapeutic Equivalence Evaluations publication (also known as the “Orange Book”), which identifies approved generic drug products and related patent and exclusivity information
Coordinates responses to generic drug shortage issues with CDER’s Drug Shortage Staff
Along with the FDA Office of Chief Counsel and CDER’s Office of Regulatory Policy, OGDP protects the integrity of OGD’s scientific determinations by ensuring that the administrative record for an application reflects OGD’s rationale and consensus.
Office of Regulatory Operations (ORO)
ORO provides oversight across all review disciplines to ensure that all generic drug review and decision-making activities are well-documented and follow a clearly defined, timely, rigorous, and scientific regulatory review process. ORO ensures that incoming ANDAs, relevant PASs, and amendments meet established quality standards for filing and labeling. ORO oversees the review of ANDAs across all disciplines to ensure OGD meets GDUFA goal dates.
ORO monitors, analyzes and improves OGD’s business processes and systems. ORO staff responds to controlled correspondence and reviews suitability petitions and ANDAs. Overall, ORO responds to more than 50,000 submissions a year.
Office of Research and Standards (ORS)
ORS leads the generic drug program in the development of scientific standards and methods for generic drug equivalence. This work includes establishing predictive and physiological models of drug product performance, drug absorption, and drug pharmacology that inform guidance development and review of in vitro, pharmacokinetic, pharmacodynamic, and clinical bioequivalence studies. ORS implements the GDUFA regulatory science program, which supports scientific research to develop pathways for generic versions of complex reference products that lack competition. The office also evaluates post-approval safety, product use, and bioequivalence of approved generic drugs.
ORS research supports guidance for developing generic forms of complex and modified-release products. Interactions with potential investigational new drug (IND) sponsors or developers may include pre-ANDA meetings and controlled correspondence regarding their individual product development. Complex and modified-release drug products require unique understanding of the scientific and clinical underpinnings of the brand-name product. Development of therapeutically equivalent generic drug products requires the joint efforts of FDA regulatory scientists and industry experts.
In 2012, Congress enacted an agreement for a generic drug user fee program, negotiated by FDA and the generic drug industry. GDUFA is part of the Food and Drug Administration Safety and Innovation Act of 2012 (FDASIA). Under GDUFA, FDA committed to performance goals, and industry agreed to pay approximately $300 million in fees each year over the five-year program. The GDUFA Commitment Letter explains the specifics of this agreement.
GDUFA performance goals are timeframes by which FDA is to take a first action on an ANDA, amendment to an ANDA, and prior approval supplements (post-approval changes requiring supplemental submission and approval). These timeframes are met by one of three actions: 1) granting an approval, 2) granting a tentative approval (in which an ANDA is ready for approval but FDA is blocked from approving due to remaining patents or exclusivities related to the reference listed drug), or 3) identifying deficiencies in an application that prevent FDA from approving it. This information is provided in a complete response letter or in a refusal to receive the application. This “refuse-to-receive” decision indicates that an ANDA is not sufficiently complete to permit a substantive review.
Table 1. Major GDUFA Performance Goals and Commitments
| Goals | Review Time | FY2015 | FY2016 | FY2017 |
|---|---|---|---|---|
Original ANDA submission | 15 months~ | 60% | 75% | 90%~ |
Tier 1 first major amendment | 10 months | 60% | 75% | 90% |
Tier 1 minor amendments (1st-3rd) | 3 months* | 60% | 75% | 90% |
Tier 1 minor amendments (4th-5th) | 6 months* | 60% | 75% | 90% |
Tier 2 amendment | 12 months | 60% | 75% | 90% |
Prior Approval Supplements | 6 months* | 60% | 75% | 90% |
ANDA teleconference requests completed | 10 business days | 200 | 250 | 300 |
Controlled correspondence+ | 2 months | 70%^ | 70% | 90% |
ANDAs, amendments and PASs in "GDUFA backlog" on Oct 1, 2012 | Act on 90% by end of FY2017 | |||
Note: Performance goals in the chart means FDA should take a “first action” (as defined above) on a certain percent of applications, etc. within the timeframes listed; it does not mean FDA should approve applications, etc. within such timeframes. For definitions of the Tier amendments, please see the GDUFA Commitment Letter.
+If no input required from clinical division
*10 months if inspection required
^4 months
~10 months
Actions on Pre-GDUFA ("Backlog") Applications
FDA committed to take a first action on 90 percent of the 2,866 ANDAs and 1,873 PASs in process with FDA or industry prior to GDUFA enactment on October 1, 2012.
This past spring, FDA met this commitment more than a year ahead of schedule. As of December 31, 2016, FDA had completed first actions on 95% of ANDAs and 93% of PASs.
Table 2 summarizes the actions FDA has taken on the ANDAs that make up the “GDUFA backlog.” Under GDUFA I, FDA committed to take a first action on more than 90% of the “GDUFA backlog.”
Table 2. Percentage of Pre-GDUFA Applications with First Actions: 10/1/2012 to 12/31/2016
(Data as of 12/31/2016)
| Actions | ANDAs | PASs |
|---|---|---|
Number with First Action* | 2,729 | 1,745 |
Percentage Complete | 95% | 93% |
Approval | 676 | 993 |
Tentative Approval | 166 | 4 |
Complete Response with an Inspection** | 1,554 | 480 |
Refuse to Receive | 68 | 2 |
Withdrawn Application | 265 | 266 |
* Numbers reflect data available at the time of report publication and may change based on refreshed counts in our tracking systems, including application status updates. These numbers are not intended for Congressional reporting purposes.
**Complete Response with an Inspection is a written FDA communication to an applicant usually describing all of the deficiencies that the agency has identified in an application that must be satisfactorily addressed before it can be approved.
Controlled Correspondence
Controlled correspondence are FDA answers to product development questions aimed to help generic drug developers. OGD responded to more than 1,800 letters and emails in 2016.
Figure 1. FY2016 Controlled Correspondence GDUFA Performance by FDA Receipt Date
(Data as of 12/31/2016)
FDA responded to more than ninety percent of controlled correspondences by the GDUFA date. The red line shows the FY2016 GDUFA metric for controlled correspondence.
*Numbers reported for FY2016 as not all GDUFA goal dates for CY2016 Controlled Correspondence have passed at the time of report publication. The FDA will report full CY2016 numbers once the data has been validated.
Improving Business Processes
FDA continued to streamline existing processes to create more efficient systems, implement written processes (e.g., standard operating procedures and manuals of policies and procedures) and enhance CDER’s Informatics Platform.
CDER Informatics Platform (The Platform)
The “Platform” is an integral part of the ANDA review processes and FDA’s ability to meet GDUFA commitments. It serves as a single integrated information technology system that coordinates and integrates ANDA review work, and maintains consistency across the CDER generic drug program. The Platform supports the review, approval, and management of original ANDAs, supplemental ANDAs, controlled correspondence related to generic drug development, facility inspections, and user fee checks. This assists with analytics and metrics reporting; goal and history tracking; process approval and task management; and management of master data, reference data, and product, sponsor, and application data.
Recent updates to the Platform include improved tracking of information requests and methods to harmonize information across the ANDA review disciplines.
Filing and Labeling Review
FDA is now able to evaluate in near-real time whether a drug applicant’s submitted application is sufficiently complete to permit a substantive review (known as “filing”). OGD issued filing decisions within 60 days for nearly all ANDAs submitted in 2016. On average, OGD makes filing decisions and notifies applicants in approximately 40 days.
Labeling review is advancing ahead of GDUFA goals. Particularly complex labeling, such as labeling for products with a REMS, is handled through a proactive, collaborative process among OGD, OSE, and CDER’s Office of New Drugs.
Staff Training and Professional Development
Iterative training for OGD employees maximizes the potential for an integrated data management system. Senior and middle managers received Lean Six Sigma training2 to increase strategic, continuous process improvement via a wide range of enhancements, from filing review to approval endorsements.
__________________________________
2 Lean Six Sigma is a fact-based, data-driven philosophy of improvement that values defect prevention over defect detection. It promotes the use of work standardization and flow.
Product-Specific Guidance Documents
OGD issues product-specific guidances to facilitate efficient filing, substantive review, and pre-market development of generic drug products. The recommendations in these guidances describe the Agency’s current thinking and expectations on development of a specific drug, and take into account the unique features of the reference listed drug (RLD) that must be incorporated into a generic version of that drug.
In 2016, OGD issued 158 new product specific guidances, many of which involved complex dosage forms, such as auto-injectors, inhalation powders, nasal sprays, topical products, and ophthalmic products.
OGD develops product-specific guidances soon after brand-name drugs are approved to ensure that patients have access to a generic drug at the earliest possible opportunity. OGD develops and issues product-specific guidance based on requests from industry and public health priorities. An OGD working group reviews requests for product-specific guidance, and makes recommendations to meet current and anticipated patient and industry needs. Requests are sent to GenericDrugs@fda.hhs.gov.
OGD revises existing product-specific guidances as new information or scientific methodology becomes available. This year, OGD revised 91 product-specific guidances. The revised draft guidances incorporate examination of emerging post-market reports of adverse events with the RLD, analysis of new studies, review of relevant literature, and reports of therapeutic inequivalence in approved ANDAs.
As of December 2016, more than 1,500 product-specific guidances are posted on FDA’s Website at https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm075207.htm.
Regulatory Guidances
In 2016, CDER published the following guidance documents for industry to assist generic drug manufacturers in their applications.
Draft Guidances:
- General Principles for Evaluating the Abuse Deterrence of Generic Solid Oral Opioid Drug Products, March 2016
- Comparability Protocols for Human Drugs and Biologics: Chemistry, Manufacturing and Controls Information, April 2016
- Assessing Adhesion with Transdermal Delivery Systems and Topical Patches for ANDAs, June 2016
- Quality Attribute Considerations for Chewable Tablets, June 2016
- Elemental Impurities in Drug Products, June 2016
- Updating ANDA Labeling After the Marketing Application for the Reference Listed Drug Has Been Withdrawn, July 2016
Final Guidances:
- Completeness Assessments for Type II API DMFs Under GDUFA, February 2016
- Environmental Assessment: Questions & Answers Regarding Drugs With Estrogenic, Androgenic, or Thyroid Activity, March 2016
- ANDA Submissions - Refuse to Receive for Lack of Justification of Impurity Limits, August 2016
- Self-Identification of Generic Drug Facilities, Sites and Organizations, September 2016
- ANDA Submissions - Prior Approval Supplements Under GDUFA, October 2016
- Generic Drug User Fee Amendments of 2012: Questions and Answers Related to User Fee Assessments, November 2016
Manuals of Policies and Procedures (MAPPs)
MAPPs document internal FDA policies and procedures, and are made accessible to the public to provide greater transparency into our operations. OGD’s MAPPs define our policy, mission and goals as they relate to generic drugs.
This year, OGD issued the following MAPPs:
- MAPP 5240.3 Rev.2, Prioritization of the Review of Original ANDAs, Amendments, and Supplements, March 2016
- MAPP 5210.5, Rev.2, Review of Investigational New Drug Applications (Bio-INDs) by the Office of Generic Drugs, October 2016
Communicating the results of regulatory science to external stakeholders, and implementing these standards in ANDA review, provides transparency and clarity that serve to improve the generic drug program.
This year, CDER continued its communication with the generic drug industry and other stakeholders through a number of events and tools, including:
- The annual Regulatory Education for Industry (REdI) Generic Drugs Forum, where FDA subject matter experts discussed with industry the best ways to communicate with the Agency, current trends in labeling and best practices, and GDUFA regulatory science.
- The REdI Pharmaceutical Quality Symposium, where FDA discussed important quality issues with small businesses.
- The Generic Pharmaceutical Association (GPhA) Annual Meeting, where FDA staff presented on GDUFA and the generic drug program.
- CDER’s Small Business & Industry Assistance newsletter (the SBIA Chronicles) and listserv (Small Biz Buzz), as well as two generic drug listservs (for GDUFA-specific updates and general generic drug updates). These tools offer educational opportunities and other updates of interest to industry.
- FDA public workshops, including “Evaluating Abuse Deterrence of Opioid Drug Products,” “Oral Absorption Modeling and Simulation,” “Substitutability of Generic Drugs: Perceptions and Reality,” and others.
FDA considers first generics to be a public health priority and expedites review of these submissions. (A list of noteworthy first generic drugs approved in 2016 is provided in this report’s appendix.) These approvals may represent the first time a generic for a particular drug has been approved, they may serve a public health need, or they may address drug shortages.
Table 3. Significant First Generic Drug Approvals in 2016
| Generic | Brand Name | Indications (Abbreviated) |
|---|---|---|
Dofetilide capsules | Tikosyn® | Atrial fibrillation/flutter |
Mometasone furoate nasal spray | Nasonex® | Nasal symptoms of seasons allergies |
Olmesartan medoxomil tablets | Benicar® | High blood pressure |
Oseltamivir phosphate capsules | Tamiflu® | Treatment of influenza |
Rosuvastatin calcium tablets | Crestor® | High cholesterol |
Sildenafil citrate tablets | Viagra® | Erectile dysfunction |
OGD maintains a complete list of first generic approvals. For full indication information, please check the Drugs@FDA online database.
Figure 2. Approvals and Tentative Approvals – CY2016
(Data as of 12/31/2016)
OGD’s regulatory science results provide new tools for industry to efficiently develop new generic drug products and for FDA to evaluate generic drug equivalence, thereby increasing access to safe and effective generic drugs.
FDA consults with and solicits input from the public, industry, and academia to develop an annual list of regulatory science initiatives specific to research on generic drugs. In May 2016 FDA held the fourth-annual public meeting on GDUFA regulatory science priorities, at which the Agency reported on the current status of the GDUFA regulatory science initiatives, and also developed the FY 2017 GDUFA regulatory science plan.
The GDUFA regulatory science plan has a large external component – FDA awarded funding to 16 new external researchers to conduct regulatory science that will complement internal activities. OGD had 87 ongoing external research collaborations at the end of 2016, because many projects awarded in previous years continued in 2016.
GDUFA research funding for new and continuing awards was distributed across the GDUFA regulatory science priority areas:
1. Post-market evaluation of generic drugs - research into monitoring methods, understanding patient perceptions of generic drugs, and verifying therapeutic equivalence via patient brand-to-generic switching studies.
These investigations provide additional data in therapeutic areas where concerns exist about the substitutability of generic drugs. They allow FDA to verify generic drug substitutability. In 2016, FDA awarded funding to assess therapeutic interchangeability between brand-name and generic products in special patient populations, and to analyze the impact of product-level, patient-level and provider-level factors on generic drug substitution.
2. Equivalence methods for complex and modified-release drug products - research into making generic versions available in all product categories, including complex drugs with unique characteristics.
FDA spends an increasing amount of time reviewing and developing policy for complex drug products and drugs having unique, modified-release characteristics. This research supports the development of guidance and policy that clarifies the ANDA pathway for complex products, such as drug-device combinations, transdermal systems, and products that contain complex mixtures and peptides. In 2016, FDA awarded funding for projects that will compare physicochemical product quality and performance attributes of ointments, identify different types of polymers used as a mixture in long-acting drug products, and develop analytical methods to profile complex drugs in urine.
3. Equivalence of locally acting products - research into new bioequivalence methods and pathways for locally acting drugs, such as inhalation, ophthalmic, or gastrointestinal drug products.
The lack of sensitive bioequivalence pathways for locally acting drug products limits the availability of generic drugs in this category. This research priority includes evaluating in vitro alternatives to comparative clinical endpoint bioequivalence studies. In 2016, the Agency made awards to evaluate pharmacokinetic profiles of dry powder inhalers, assess the differences in response among individuals of small airway delivery for orally inhaled drug products, and assess the dermal pharmacokinetics by microdialysis and microperfusion techniques.
4. Therapeutic equivalence evaluation and standards - support the evolution of risk-based equivalence and product quality standards to ensure therapeutic equivalence across all dosage forms and routes of delivery.
FDA continues to prioritize research on abuse-deterrent formulations, narrow therapeutic index drugs and equivalence of modified-release solid oral dosage forms. In 2016, the Agency made new awards to develop a mechanism-based absorption model to predict pharmacokinetic profiles of supersaturating formulations, and to evaluate formulation dependence of drug interaction with proton pump inhibitors for oral modified-release products.
5. Cross-cutting computational and analytical tools - essential to developing a modern ANDA review process.
Research priorities for advanced analytical methods include developing methods that characterize peptides and other complex mixtures that evaluate particle size, surface chemistry and gene expression for impurities or immunogenicity. Modeling and simulation tools that FDA will investigate include pharmacodynamic models or clinical trial simulation, systems biology, and quantitative risk modeling. In 2016, FDA awarded funding to evaluate model-based bioequivalence statistical approaches for sparse design pharmacokinetic studies, and to develop an algorithm for population-based statistical analysis in physiologically based pharmacokinetic models.
In 2016, GDUFA funded more than $54 million in research programs. In keeping with FDA’s commitment to promote quality science and clinical relevance, updates on our work are available in more than 100 published scholarly articles, presentations, posters, and book chapters.
Significant 2016 Research Accomplishments
A GDUFA-funded study to develop new methods for evaluation of topical bioequivalence demonstrated for the first time that clinical dermal open-flow microperfusion was a viable cutaneous pharmacokinetic approach to compare local bioavailability and to support bioequivalence evaluations for a topical locally-acting dermatological product1.
In addition, the results of two bioequivalence studies3 with lamotrigine immediate release tablets, conducted in patients with epilepsy under clinical conditions, supported the validity of the FDA bioequivalence standards. As a result, the American Epilepsy Society (AES) rescinded its 2007 position statement opposing generic substitution of antiepileptic drugs and acknowledged in a statement that drug formulation substitution with FDA-approved generic products reduces cost without compromising efficacy.4
____________________________________________
1 Clin Pharmacokinet. 2016 Aug 18. [Epub ahead of print] Open Flow Microperfusion as a Dermal Pharmacokinetic Approach to Evaluate Topical Bioequivalence. Bodenlenz M1, Tiffner KI1, Raml R1, Augustin T1, Dragatin C1, Birngruber T1, Schimek D1, Schwagerle G2, Pieber TR1,2, Raney SG3, Kanfer I4,5, Sinner F6,7. DOI: 10.1007/s40262-016-0442-z
3 Ting, TY, Jiang W, Lionberger R, Wong J, Jones JW, Kane MA, Krumholz A, Temple R, Polli JE. Generic lamotrigine versus brand-name Lamictal bioequivalence in patients with epilepsy: A field test of the FDA bioequivalence standard. Epilepsia, 56: 1415–1424. doi: 10.1111/epi.13095 (2015) Privitera MD, Welty TE, Gidal BE, Diaz FJ, Krebill R, Szaflarski JP, Dworetzky BA, Pollard JR, Elder EJ Jr, Jiang W, Jiang X, Berg M. Generic-to-generic lamotrigine switches in people with epilepsy: the randomised controlled EQUIGEN trial.Lancet Neurol;15(4):365-72 (Apr 2016)
4 Vossler DG, Anderson GD, Bainbridge J. AES Position Statement on Generic Substitution of Antiepileptic Drugs. Epilepsy Current. 16:209-211 (2016)
2016 Office of Generic Drugs (OGD) Annual Report (PDF - 1.18 MB)
We Welcome Your Feedback
we work to meet GDUFA goals.
Office of Generic Drugs
Center for Drug Evaluation and Research
U.S. Food and Drug Administration
10903 New Hampshire Avenue, Building 75
Silver Spring, Maryland 20993
GenericDrugs@fda.hhs.gov
(240) 402-7920