Perspective: FDA’s Progress on Review of Tobacco Product Applications Submitted by the Sept. 9, 2020 Deadline
By Mitch Zeller, Director of the FDA’s Center for Tobacco Products (CTP)
February 16, 2021
Per a court order, premarket applications for many new tobacco products, including e-cigarettes, certain cigars, and hookah products, currently on the market were due to FDA by Sept. 9, 2020. Also consistent with a court order, products for which applications were submitted by the Sept. 9 deadline may remain on the market for up to a year pending FDA review, although they remain subject to FDA enforcement. This piece is a follow-up to our August perspective piece and aims to provide an update on the progress we have made on the processing and review of these applications. 1
Background
Following the Sept. 9 premarket application deadline for certain deemed new tobacco products on the market as of Aug. 8, 2016, FDA’s job is to process, review, and take action on a massive number of applications for products that are currently on the market. Premarket review of new tobacco products is a critical part of how we carry out our mission to protect the public—especially kids—from the harms associated with tobacco use.
This undertaking represents a major milestone for tobacco product regulation and for public health as a whole. By implementing the premarket review requirement for new electronic nicotine delivery systems (ENDS) and other “deemed” new tobacco products, such as hookah and pipe tobacco, we are taking steps to transform the marketplace toward one where deemed new tobacco products available for sale will have undergone careful, science-based review and oversight by the FDA. 2
We have worked for several years to prepare for premarket review of a large number of deemed products. These efforts included improving information technology systems, engaging with stakeholders, significantly increasing hiring, streamlining review procedures, and providing and promoting guidance and resources to inform industry.
As anticipated, we received thousands of tobacco product submissions covering millions of tobacco products, the majority of which came in very close to the Sept. 9 deadline. Furthermore, the submissions varied substantially in number of tobacco products contained in each submission, size, format and organization. However, despite these challenges, due to our preparations and continued engagement with stakeholders, the “intake” of the large number of submissions went smoothly; our IT systems performed as designed and were able to handle both a high number of submissions within a short period of time and extremely large individual submissions. Now, the initial “intake” of submissions is nearly complete, and the acceptance, filing, and substantive review of applications is underway.
In an August perspective piece, I pledged that the agency would keep interested stakeholders updated on the agency’s progress. We are now at a point in the review of these applications where we can share some updates on our progress.
Processing and Reviewing Applications
Receipt and Processing of Submissions
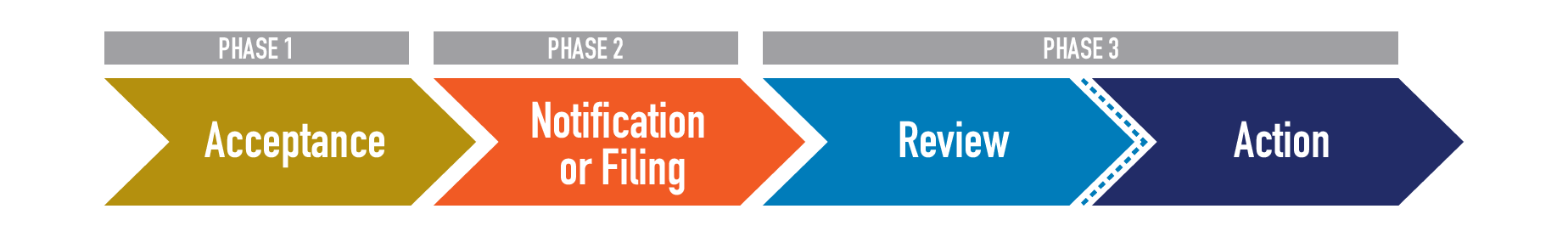
After a firm submits their application(s) to the FDA, the agency goes through a number of Processing steps to properly receive and prepare it for the review process (e.g., acceptance review, filing review, substantive review). This includes physical or electronic “intake” of the submission and determining the type and number of applications contained in the submission. For example, this is when we determine if a submission is a Premarket Tobacco Product Application (PMTA) or Substantial Equivalence (SE) Report and how many individual applications for tobacco products are included within the submission. This is also when FDA ensures that the files are safely viewable by conducting virus scans on hard drive submissions and unzipping any zipped files. Lastly, FDA uploads all the files into internal review systems to prepare them for the next step in the review process.
We have seen significant variety in the number of tobacco products included in each submission, so each and every submission package is being carefully assessed. For example, some applicants provided information on one product per submission while other applicants provided information for all of the company’s products within one submission. In addition, the submissions arrived in many different formats, including electronic submissions, paper submissions, and mixed media (flash drives, hard drives or CDs, sometimes combined with paper), and varied widely in their organization and presentation of information. We also received several duplicate submissions; for example, companies submitted an electronic version and a paper version of the same application. Lastly, while some firms used a spreadsheet made available by FDA to enable faster processing, most firms used other means to present the information. This impacted our ability to more quickly process the information provided, as it required our staff to manually enter the product information into our systems.
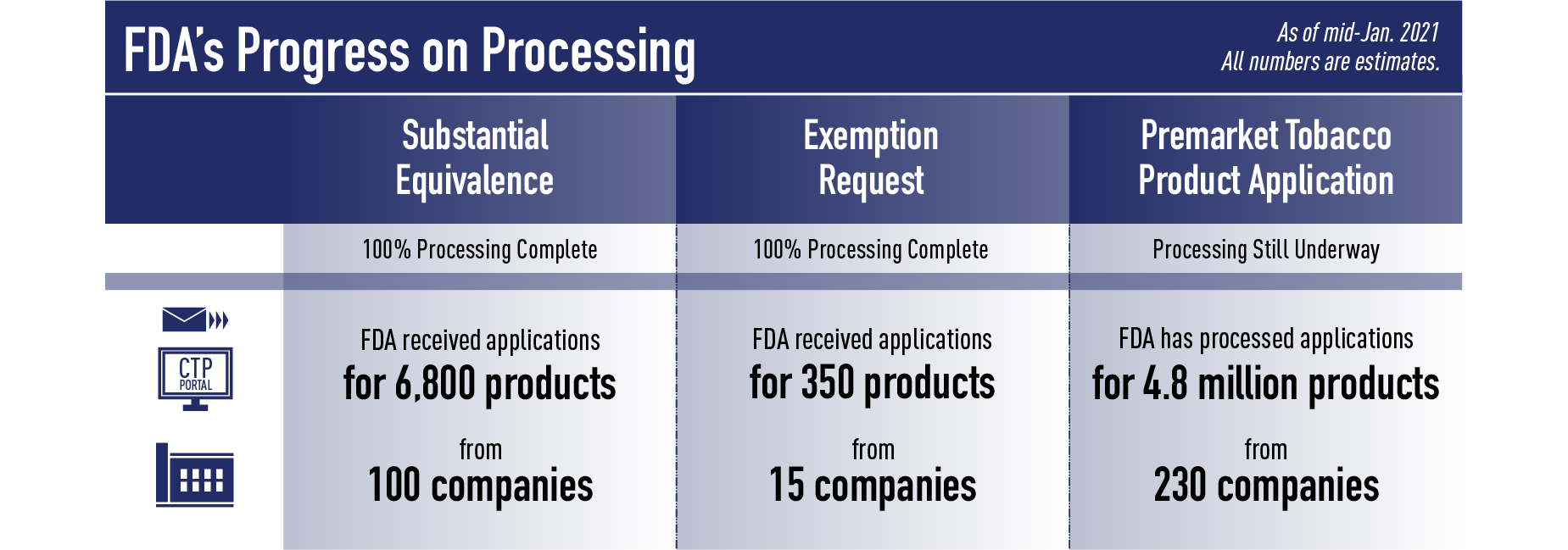
Recently, we completed the Processing step of ALL Exemption from Substantial Equivalence Requests (EX REQ) and ALL Substantial Equivalence (SE) Reports submitted by the Sept. 9 deadline. For the EX REQ pathway, we received applications for about 350 products from about 15 companies. For the SE pathway, we received applications for about 6,800 products from about 100 companies.
For PMTAs, as of mid-January 2021, the agency has completed the Processing step of applications for more than 4.8 million products from over 230 companies. During Processing, FDA found that the PMTAs posed additional challenges due to the size, complexity and diversity of the submissions; for example, some firms provided separate submissions for each section of the tobacco product application, such as submitting the clinical information separately from product identification and manufacturing information, while others included up to tens of thousands of products within a submission. One firm submitted information on more than 4 million tobacco products within a single submission. The amount of content in each submission also greatly varied, with some applications including up to 2,000,000 files where each file contains multiple pages of content for FDA to review.
Given the high level of public interest in these submissions, we’d hoped to be able to share a list of products submitted under all three pathways at once. However, we have not completed Processing all of the applications submitted through the PMTA pathway. Because we want to provide as much of an update as possible, we are therefore sharing the SE and EX REQ list and providing a PMTA update at this time.
While processing PMTA submissions, the Agency continues to enforce the law. As previously stated in FDA’s enforcement priorities, after Sept. 9, 2020, FDA is prioritizing enforcement against any ENDS product that continues to be sold and for which the agency did not receive a product application. Additionally, for deemed tobacco products—other than ENDS or “premium cigars”—that do not have premarket authorization, FDA will make enforcement decisions on a case-by-case basis and intends to prioritize enforcement based on the likelihood of youth use or initiation to make the most efficient use of its resources (FDA is currently enjoined from enforcing the premarket requirements for products that meet the definition of “premium cigar” in the court’s order).
In January 2021, FDA issued the first set of warning letters to firms who have not submitted premarket applications to FDA and are continuing to sell or distribute unauthorized ENDS after Sept. 9, 2020. To date, FDA has sent warning letters to 30 firms who manufacture and operate websites selling electronic nicotine delivery system (ENDS) products, specifically e-liquids, which lack premarket authorization.
We are continuing to process packages submitted by the Sept. 9 deadline and aim to share the final PMTA numbers as soon as possible.
Acceptance and Filing
Once a submission package is processed, the individual product applications within the submission proceed to the review process.
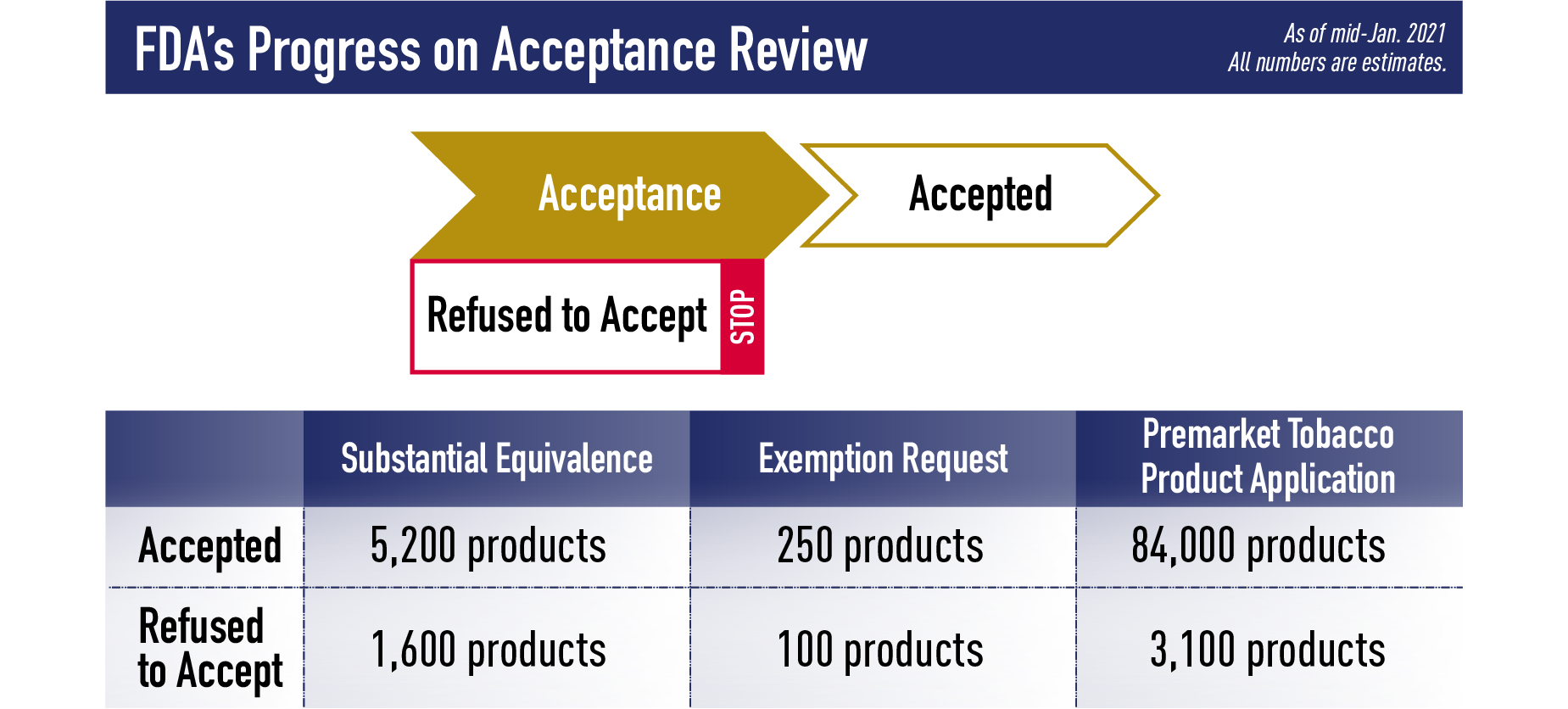
Phase 1 in the review process is to conduct an application’s Acceptance review. This review ensures the product falls under CTP’s jurisdiction and confirms that basic requirements of an application are met. If an application is Accepted, it will move on to the Notification or Filing stage (based on which pathway it was submitted through). During the Acceptance review, FDA may also Refuse to Accept (RTA) a premarket application if it contains certain deficiencies. Examples of deficiencies that lead to an RTA include: if the submission does not pertain to a tobacco product, if it is not in English or does not contain complete English translations, if it is in an electronic format FDA cannot process, read, review, and archive, or if the submission does not include an environmental assessment or valid claim of categorical exclusion. If a product that is currently on the market is part of an application that receives an RTA, that product must be removed from the market or risk FDA enforcement action.
As of mid-January 2021, of the applications submitted by Sept. 9, we have accepted applications for about 5,200 products and refused to accept applications for about 1,600 products submitted through the SE pathway, and we have accepted applications for about 250 products and refused to accept applications for about 100 products submitted through the EX REQ pathway. Finally, we have accepted applications for about 84,000 products and refused to accept applications for about 3,100 products submitted through the PMTA pathway.
Phase 2 varies by application type. All accepted PMTAs proceed through filing review, which ensures the application contains all the items required under Section 910(b)(1), including, but not limited to, full reports of all information concerning investigations which have been made to show the health risks of the tobacco product and whether the tobacco product presents less risk than others; full statements of the components, ingredients, additives, and properties, and of the principle or principles of operation; and full descriptions of the methods used in, and the facilities and controls used for, the manufacture, processing, and, when relevant, packing and installation of, the tobacco product. Applications that meet the requirements will be filed and enter the substantive review queue. Those that do not meet the requirements will be the subject of a Refuse to File (RTF) letter.
As of mid-January 2021, of the applications submitted by Sept. 9, we have filed applications for about 29,000 products and refused to file applications for about 1,650 products submitted through the PMTA pathway.
SE Reports and EX REQs enter into the notification step for Phase 2. During this phase, the review teams are assembled, and appropriate processes are kicked off to determine predicate product eligibility.
More detailed information for each of the application types can be found on the pathway-specific pages for PMTAs, SE Reports and EX REQs.
Following the issuance of an RTA or RTF letter, the FDA regulatory health project manager (RHPM) places a call to the applicant notifying them of the letter issued and offering a courtesy copy. To help improve transparency of the review process, following completion of the acceptance and filing phases, applicants whose submissions are accepted (and filed for PMTAs) are notified by phone of the initiation of the substantive review phase.
Review/Action
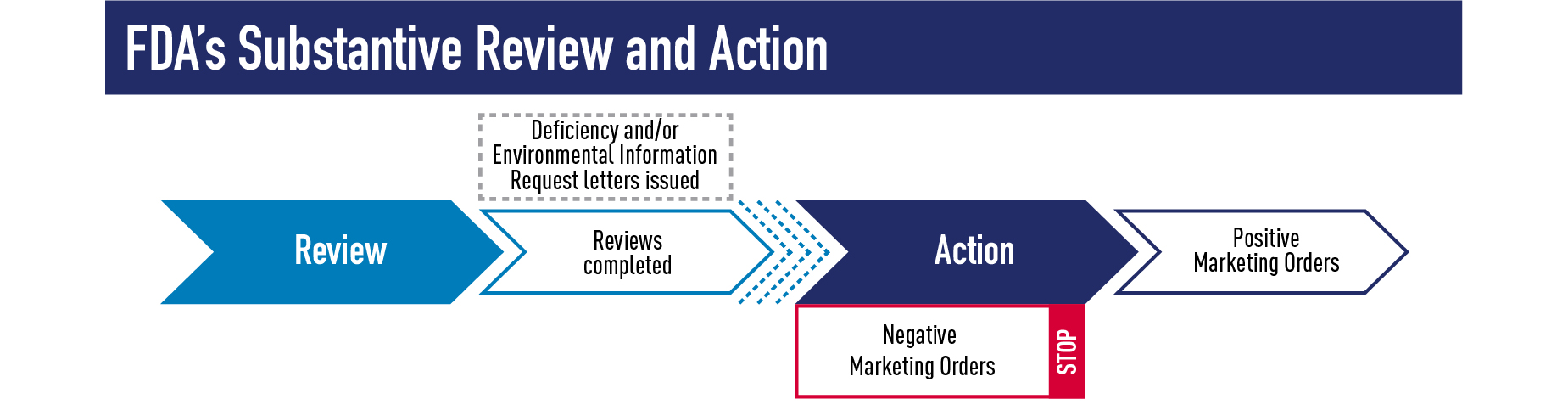
The substantive review phase (Phase 3) includes evaluation of the scientific information and data in an application. This is the longest and most thorough phase of FDA’s review process and often results in follow-up questions and conversations with the applicant. Substantive review of a PMTA, for example, includes a multi-disciplinary review of a product’s engineering, chemistry, toxicology, behavioral and clinical pharmacology, microbiology, individual health impact, and population health impact. In addition, inspections of manufacturing sites or clinical investigations may be necessary. Before a final decision is made, substantive review often results in a Deficiency Letter or Environmental Information Request Letter requesting additional information from the applicant before FDA can make a final marketing decision. However, such letters are not always needed, and substantive review may also directly result in the final decision of a product receiving a positive marketing order, such as an SE, Found Exempt, or Marketing Granted order; or a negative marketing order, such as a Not Substantially Equivalent (NSE), Not Exempt (NEX) or Marketing Denial order.
We are working expeditiously and have already completed substantive review for some SE Reports and EX REQs submitted by Sept. 9, 2020. As of mid-January 2021, FDA has already issued over 50 SE and 125 EX marketing orders for tobacco products, 3 which includes actions on applications subject to the Sept. 9 deadline that were submitted earlier last year. For EX REQs, if an Exempt order letter was issued, an Abbreviated Report must be submitted to FDA and 90 days passed since FDA’s receipt of the Abbreviated Report before legally marketing a new tobacco product. FDA has not yet issued NSE or NEX orders on applications submitted by the Sept. 9 deadline; instead in most cases FDA is issuing a Deficiency letter to allow one opportunity for applicants to correct deficiencies in their submissions.
We have also commenced substantive review on hundreds of products submitted through the PMTA pathway. FDA will notify a firm if one of their applications is moving into the substantive review phase.
Allocating Review Resources and Determining Review Order for Applications Submitted by Sept. 9, 2020
Due to the large number of applications moving into review at the same time, the novelty of this review, the finite nature of our review resources, and the necessarily rate-limiting effects of ensuring consistency across reviews, FDA developed a process to determine the review order for the applications. This process applied to all applications that were submitted to CTP by Sept. 9, 2020, including originally-regulated products such as cigarettes and smokeless tobacco and deemed products that are not currently marketed.
The first step in the process is for FDA to classify an application as an SE Report, EX REQ, or PMTA based on the information provided by the applicant. The review order for the applications was then determined as follows:
SE Reports and EX REQs
For applications classified as SE Reports and EX REQs, review order was determined using randomization by manufacturer. 4 Using a basic random number generator, FDA assigned a number to the manufacturers that submitted at least one application to determine the order for entering acceptance review and subsequent review phases (e.g. notification, substantive review). At the substantive review, if the manufacturer submitted a number of products that exceeded the capacity of the scientific review team, FDA assigned a second randomly-generated number to each product in each submission to determine the order of the products. The products that are not assigned to the review team will remain in queue until all of the manufacturers with timely, accepted applications have had some products enter the substantive review phase once; this ensures that every manufacturer has an opportunity for some of its products to enter substantive review. The randomly-generated numbers stay with the application for the individual product and continue to determine its place in the queues throughout the review process.
PMTAs
For applications classified as PMTAs, the review order for most of the products is also determined using the same randomization process used for the SE Reports and EX REQs. Though the number of PMTAs continues to grow as applications are processed, nearly all of the PMTAs submitted will enter the review queues through a randomized process. However, due to the large number of ENDS products currently marketed and for which we anticipated receiving submissions, FDA decided to dedicate a portion of its resources to reviewing the products that account for most of the current market.
The continued marketing of these products has the potential to have the greatest public health impact—either positively or negatively—as they hold the largest overall market share and therefore likely used by the largest number of people. For this reason, FDA pulled several applications into a separate review queue and dedicated resources to their review. By identifying and ensuring first review of these applications, we believe we can achieve the greatest public health impact most quickly. If FDA finds that a widely-used, currently marketed product does not meet the standard in the law for marketing, the Agency will not grant a marketing order and the product must be removed from the market. Conversely, if FDA finds that a widely-used, currently marketed product does meet the standard in the law for marketing, the Agency will grant a marketing order and the product may remain on the market subject to the conditions in the order. In either case, earlier review ensures a faster transition to a marketplace of products that have been scientifically reviewed for their impact on public health.
We are working to review applications as quickly as possible. However, given the unprecedented number of applications and other factors discussed above, the likelihood of FDA reviewing all the applications by Sept. 9, 2021 is low. We will continue to allocate our resources with the goal of working as quickly as possible to transition the current marketplace for deemed products to one in which all products available for sale have undergone a careful, science-based review by FDA. We will focus resources on products where scientific review will have the greatest public health impact, based on their market share, while also committing to providing an opportunity for review to all companies regardless of size, prior to Sept. 9, 2021, at which time they risk FDA enforcement per FDA’s guidance.
Maintaining Transparency
Updates to this communication were posted on May 20, 2021 and can be found here
Posting of the “List of Deemed New Tobacco Products with Timely Applications”
We understand the great interest from the public about what product applications were submitted, which is why we have stated our intention to post a list of timely-submitted applications in accordance with applicable laws. This includes, for example, verifying with each individual applicant the dates of initial marketing and current marketing status of each product submitted by the Sept. 9 deadline.
Since September, we have been contacting applicants to verify this key information, if it was not included in their submissions. We have now contacted applicants for all product applications received through the SE and EX REQ pathways and have posted a list of products that were on the market as of Aug. 8, 2016, are currently marketed, and are the subject of a pending, timely applications submitted through these two pathways.
The list includes over 2000 products, including about 1100 cigars, over 330 pipe tobacco products, and over 660 waterpipe tobacco products. The list is based on information received from companies and does not include entries for companies that did not verify the marketing status of their products before the time of posting.
Separately, we continue to work on processing submissions and verifying the dates of initial marketing and current marketing status of products received through the PMTA pathway. We have already verified this information for around 86,000 products received through the PMTA pathway. Due to the size and volume of the PMTA submissions and the variable quality, format and presentation of these submissions, processing these submissions and verifying this information will take more time. We will post information on products submitted through the PMTA pathway after verifying the information noted above.
It is important to note that this is not a comprehensive list intended to cover all currently marketed deemed tobacco products that a firm generally might manufacture, distribute, or sell without risking FDA enforcement, per FDA’s guidance. For example, the list will not contain products from companies that did not verify their information before the time of posting, products that are subject to a positive marketing order from the FDA, products with a pending submission that was not submitted by the Sept. 9, 2020, deadline, or products that were commercially marketed in the United States as of Feb. 15, 2007 (grandfathered tobacco products) and have not been modified. With respect to deemed tobacco products, FDA expects this grandfathered status to apply to many cigars, hookah tobacco, and pipe tobacco products.
In addition, per a court ruling issued Aug. 19, 2020, FDA is currently not enforcing the premarket review requirement against manufacturers of “premium cigars,” as defined in that court’s ruling, that did not submit premarket applications for these products by the Sept. 9 deadline. However, either before or after Aug. 19, 2020, a manufacturer could have submitted an application for a product that is a “premium cigar” as defined in the court’s order. This list does not categorize whether a product meets this definition, and FDA did not consider whether any cigar products fell within the “premium cigar” definition used in the court order during creation of these lists. As with other products, where a manufacturer did not submit an application for a “premium cigar” by Sept. 9, 2020, or did not verify the marketing status of its products, the product would not be on this list. Furthermore, as manufacturers are not required to submit a SE or EX REQ for products that have different names and/or labels if the products are physically identical, some products on the list may also be marketed under a different name and/or label.
Ultimately this list is only one source of information related to which products may be the subject of pending applications before the Agency; retailers should discuss with their suppliers about the current status of any particular tobacco product application or any product’s marketing status. 5
Expanding Publicly Available Data and Metrics
To help fulfill our commitment to transparency during this process, starting today, we will be posting expanded data on our new Tobacco Product Application Metrics & Reporting webpage. This webpage will be updated regularly and will provide more detailed updates of the agency’s progress on premarket application review than previously available. For example, for the first time, we will be posting all metrics both by pathway (SE, EX REQ, and PMTA) and by product category type (e.g., cigarette, ENDS, cigars, smokeless).
We will be providing updates every other month (bi-monthly) on intermediate actions such as the number of products for which applications were received and accepted and the number of products for which applications were filed. We will continue to post updates on the number of products for which FDA has taken actions, such as RTA and RTF.
We will also post aggregate data on the completion of each step throughout the substantive review phase and the actions taken—including the issuance of positive and negative marketing orders—on a quarterly basis. Any tobacco products that receive authorization from FDA will continue to be listed on our Tobacco Product Marketing Orders page.
These bi-monthly and quarterly updates will include a much wider range of metrics along the review process than we have provided in the past. This will allow the public and interested stakeholders to track and monitor the progress being made through the process for each of the application types and product categories.
We understand and appreciate the interest in our application review efforts. Today’s update on the progress being made in the review of tobacco product applications submitted by the Sept. 9 deadline is part of our ongoing commitment to being transparent about this important work. We will continue to provide updates on the agency’s progress on a regular basis. For the latest updates, please check the metrics and reporting and the products list pages regularly and sign up for email updates from CTP.
- 1 Readers are encouraged to review the August perspective piece for additional background on the relevant regulatory history as well as other information on product review.
- 2Per a court ruling issued Aug. 19, 2020, FDA will not enforce the premarket review requirement against manufacturers of “premium cigars,” as defined in that ruling, while this order is in effect. Read the web statement. Additionally, some deemed tobacco products that have “grandfathered status” because they were commercially marketed in the United States as of Feb. 15, 2007, do not need to submit premarket applications (unless the products were since modified).
- 3The numbers provided for SE and EX REQ marketing orders are for a combination of originally-regulated and deemed products.
- 4Regardless of tobacco product type
- 5 In addition to the reasons stated in the text, products were also excluded from the list if for example a product received a “Refuse to Accept” or “Refuse to File” letter from FDA, if the application was withdrawn by the applicant, or if the application was administratively closed prior to the posting of the list.