COMPANY ANNOUNCEMENT
SD BIOSENSOR, Issues Notification of Voluntary Recall of ‘STANDARD Q COVID-19 Ag Home Test’
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Medical Devices
- Reason for Announcement:
-
Recall Reason Descriptionillegally imported into the United States
- Company Name:
- SD Biosensor, Inc.
- Brand Name:
-
Brand Name(s)SD Biosensor, Inc.
- Product Description:
-
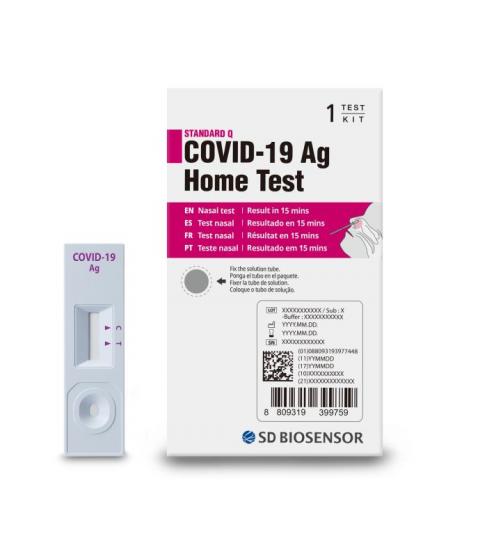
Product DescriptionSTANDARD Q COVID-19 Ag Home Test
Company Announcement
January 31, 2022. SD Biosensor, Inc., a global in-vitro diagnostics company, is voluntarily recalling its STANDARD Q COVID-19 Ag Home Test in the United States, due to confirmed reports that the test kits were illegally imported into the United States. The STANDARD Q COVID-19 Ag Home Test is not authorized, cleared or approved by the U.S. Food and Drug Administration (FDA) for distribution or use in the United States. While there is no known distribution of these tests directly to consumers, SD Biosensor, Inc. is issuing this voluntary recall out of an abundance of caution.
STANDARD Q COVID-19 Ag Home Test
STANDARD Q COVID-19 Ag Home Test is a rapid chromatographic immunoassay for the qualitative detection of SARS-CoV-2 nucleocapsid antigen present in human nasal sample. It provides only an initial screening test result. The result of this test should not be the sole basis for the diagnosis; confirmatory testing is required.
Recommendations for Consumers and Test Users
In the unlikely event that consumers in the United States encounter the ‘STANDARD Q COVID-19 Ag Home Test’, they are encouraged to discard and avoid any use of the test, as it has not been authorized, cleared or approved by the FDA for use in COVID-19 testing and diagnosis in the United States. Consumers that have used the test are strongly encouraged to consider retesting with an FDA authorized or cleared test.
Measures to Prevent Further Illegal Importation
SD Biosensor, Inc. considers illegal importation to be a grave matter. In addition to issuing a voluntary recall for the ‘STANDARD Q COVID-19 Ag Home Test’, SD Biosensor, Inc. has initiated an investigation to determine how the product was illegally imported into the United States. Distributors or individuals who illegally imported the products initially sold outside the United States will be ordered to stop the illegal activity and initiate an immediate product recall.
SD Biosensor, Inc. is taking appropriate measures to prevent further attempts at illegal importation of unauthorized tests by strengthening contract terms and their enforcement with its distributors. In addition, the company announced publicly that if such illegal importations are discovered in the future, the responsible individuals/distributors will face strict legal action and liabilities for damages.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178