COMPANY ANNOUNCEMENT
Mylan Pharmaceuticals Inc., a Viatris Company, Conducting Voluntary Nationwide Recall of One Batch of Insulin Glargine (Insulin glargine-yfgn) Injection, 100 units/mL (U-100), Due to the Potential for a Missing Label in the Batch
This recall has been completed and FDA has terminated this recall.
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason DescriptionLabel may be missing on some vials
- Company Name:
- Mylan Pharmaceuticals, Inc. a Viatris Company
- Brand Name:
-
Brand Name(s)Mylan
- Product Description:
-
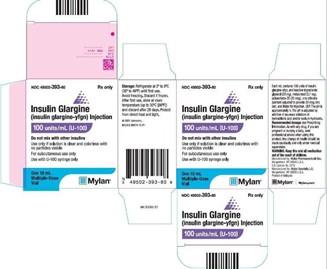
Product DescriptionInsulin Glargine (Insulin glargine-yfgn) Injection
Company Announcement
FOR IMMEDIATE RELEASE - PITTSBURGH – April 12, 2022 – Mylan Pharmaceuticals Inc., a Viatris company, is voluntarily recalling one batch of its Insulin Glargine (Insulin glargine- yfgn) Injection, 100 units/mL (U-100), which is packaged in a 10 mL vial that is inside a carton. This product is not the branded Semglee vial but the unbranded Insulin Glargine- yfgn vial. This batch is being recalled due to the potential for the label to be missing on some vials. The product information, batch number and expiry date information are present on the carton.
This batch was manufactured by Biocon Sdn. Bhd. and was distributed by Mylan Specialty L.P. in the US between December 9, 2021, and March 4, 2022. The recalled batch is as follows:
| NDC # | Name and Strength | Size | Batch# | Expiry |
|---|---|---|---|---|
| 49502-393-80 | Insulin Glargine (Insulin glargine-yfgn) Injection, 100 units/mL (U-100) |
10 mL vial | BF21002800 | Aug 2023 |
Risk Statement: For patients receiving treatment with more than one type of insulin (e.g., both short and long-acting insulin), a missing label on Insulin Glargine vials could lead to a mix-up of products/strengths, which may result in less optimal glycemic control (either high or low blood sugar) which could result in serious complications. To date, no adverse events related to this recall have been received for this product.
This recall does not pertain to the branded interchangeable biosimilar, Semglee® (insulin glargine-yfgn) injection but to the unbranded interchangeable biosimilar Insulin Glargine-yfgn vial.
The product is a long-acting human insulin analog indicated to improve glycemic control in adults and pediatric patients with type 1 diabetes mellitus and in adults with type 2 diabetes mellitus.
The company has initiated the recall of batch BF21002800 and notified its distributors and retailers by letter and is arranging for return of all recalled products. Following are actions for wholesalers, retailers and consumers:
- Wholesaler: Immediately examine your inventory, quarantine, and discontinue distribution of the batch subject to recall. In addition, if you have further distributed the product, please identify all customers, including retail level customers, and provide a list of customers via Microsoft excel file to mylan5889@sedgwick.com within 5 business days. Sedgwick will notify your retail level customers that received the affected batch.
- Retailer: Immediately examine your inventory, quarantine, and discontinue distribution of this batch.
- Consumer: If you have an unlabeled product, please contact Sedgwick at 1-888-912-7084 for the documentation packet to return product to Sedgwick.
Consumers with questions regarding this recall can contact Viatris Customer Relations by 1-800-796-9526 or customer.service@viatris.com, Monday through Friday from 8 a.m. – 5 p.m. EST. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
About Viatris
Viatris Inc. (NASDAQ: VTRS) is a new kind of healthcare company, empowering people worldwide to live healthier at every stage of life. We provide access to medicines, advance sustainable operations, develop innovative solutions and leverage our collective expertise to connect more people to more products and services through our one-of-a-kind Global Healthcare Gateway®. Formed in November 2020, Viatris brings together scientific, manufacturing and distribution expertise with proven regulatory, medical, and commercial capabilities to deliver high-quality medicines to patients in more than 165 countries and territories. Viatris' portfolio comprises more than 1,400 approved molecules across a wide range of therapeutic areas, spanning both non-communicable and infectious diseases, including globally recognized brands, complex generic and branded medicines, a portfolio of biosimilars and a variety of over-the-counter consumer products. With a global workforce of approximately 37,000, Viatris is headquartered in the U.S., with global centers in Pittsburgh, Shanghai and Hyderabad, India. Learn more at viatris.com and investor.viatris.com, and connect with us on Twitter at @ViatrisInc, LinkedIn and YouTube.
Contacts:
Media:
+1.724.514.1968
Communications@viatris.com
Jennifer Mauer
Jennifer.Mauer@viatris.com
Matt Klein
Matthew.Klein@viatris.com
Investors:
+1.724.514.1813
InvestorRelations@viatris.com
Bill Szablewski
William.Szablewski@viatris.com
Company Contact Information
- Consumers:
- Viatris Customer Relations
- 1-800-796-9526
- customer.service@viatris.com
- Media:
- Jennifer Mauer
- +1-724-514-1968
- Jennifer.Mauer@viatris.com