COMPANY ANNOUNCEMENT
Hospira, Inc. Issues A Voluntary Nationwide Recall For One Lot of Vancomycin Hydrochloride Injection, USP 1.5g/vial, Due To The Presence of Visible Glass Particulates
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason DescriptionPresence of Visible Glass Particulates
- Company Name:
- Hospira, Inc.
- Brand Name:
-
Brand Name(s)Hospira, Inc.
- Product Description:
-
Product DescriptionVancomycin Injection
Company Announcement
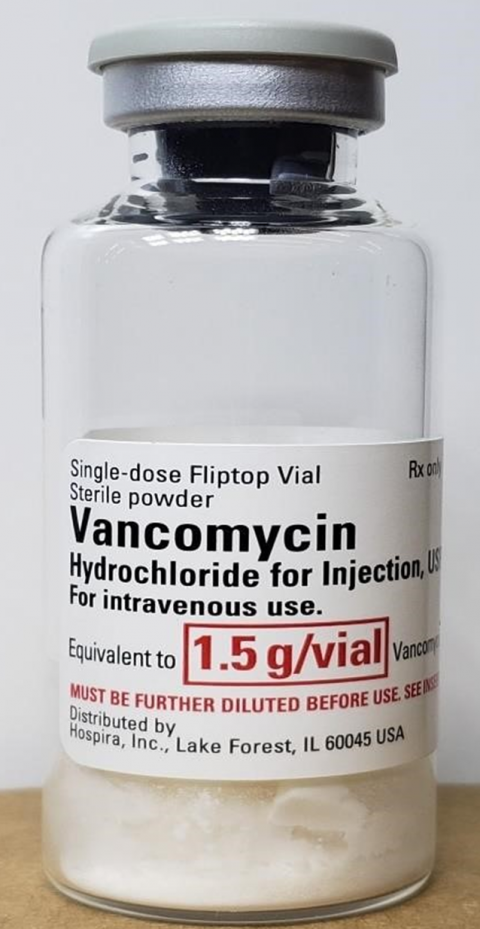
December 22, 2022 - NEW YORK, NY., Hospira, Inc., a Pfizer company, is voluntarily recalling one lot of Vancomycin Hydrochloride Injection, USP, 1.5 g/vial Single Dose Fliptop Vial, lot 33045BA, to the user level due to two visible glass particulates observed in a single vial.
If administered intravenously, patients may experience adverse events such as local irritation or swelling, vasculitis/ phlebitis, antigenic or allergic reactions, and microvascular obstruction, including pulmonary embolism. If administered orally or via a nasogastric tube, there may be the potential for gastrointestinal trauma. The risk is reduced by the possibility of detection, as the label contains a statement directing the healthcare professional to visually inspect the product for particulate matter and discoloration prior to administration.
To date, Pfizer has not received reports of any adverse events related to this recall.
Vancomycin Hydrochloride is an antibiotic indicated for the treatment of serious or severe infections caused by susceptible strains of methicillin-resistant staphylococci. Vancomycin Hydrochloride is effective in the treatment of staphylococcal endocarditis, septicemia, bone infections, lower respiratory tract infections, and skin and skin-structure infections. It is used in penicillin-allergic patients, and also for patients who cannot receive or who have failed to respond to other antimicrobials, including penicillin or cephalosporin agents, and for infections caused by vancomycin-susceptible organisms that are resistant to other antimicrobials.
The NDC, Lot Number, Expiration Date, and Configuration details for Vancomycin HCl for injection, USP are indicated below. The product was distributed nationwide to wholesalers/hospitals/ institutions in the United States and Puerto Rico from June 23, 2022 through September 19, 2022.
|
Product |
NDC |
Lot |
Expiration |
Presentation |
Configuration/ |
|---|---|---|---|---|---|
| Vancomycin Injection, USP, Single- Dose Fliptop Vial |
Vial: 0409-3515-11 Carton: 0409-3515-01 |
33045BA | 1SEP2023 | 1.5 g/Vial | 10 units/carton, 10 cartons/case |
Pfizer places the utmost emphasis on patient safety and product quality at every step in the manufacturing and supply chain process. Pfizer has notified direct consignees by letter to arrange for return of any recalled product.
Wholesalers, hospitals, institutions, and doctors with an existing inventory of the lot, which is being recalled, should discontinue use, stop distribution and quarantine immediately. If you have further distributed the recalled product, please notify your accounts and/or any additional locations which may have received the recalled product. Hospitals/Institutions should inform Healthcare Professionals in your organization of this recall. For additional assistance, call Sedgwick Inc. at 1-800-805-3093 between the hours of 8 a.m. to 5 p.m. ET, Monday through Friday.
Healthcare Professionals with questions regarding this recall can contact Pfizer using the below information.
|
Contact Center |
Contact Information |
Area of Support |
|---|---|---|
| Pfizer Medical Information | 1-800-438-1985, option 3 (9am to 5pm ET Monday through Friday) www.pfizermedinfo.com |
For medical questions regarding the product |
| Pfizer Drug Safety | 1-800-438-1985, option 1 (24 hours a day; 7 days a week) |
To report adverse events and product complaints |
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Company Contact Information
- Consumers:
- Sedgwick Inc.
- 1-800-805-3093
- Media:
- Media Relations
- +1-212-733-1226
- PfizerMediaRelations@pfizer.com