COMPANY ANNOUNCEMENT
Asiaticon SA de CV Issues Voluntary Nationwide Recall of V-Klean Hand Sanitizer Gel, Medically Minded Hand Sanitizer Gel and Protz Real Protection Antibacterial Hand Sanitizer Due to Potential Presence of Methanol (Wood Alcohol) and Subpotent Ethanol Levels
This recall has been completed and FDA has terminated this recall.
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason DescriptionPotential presence of methanol (wood alcohol) and subpotent ethanol levels
- Company Name:
- Asiaticon SA de CV
- Brand Name:
-
Brand Name(s)V-Klean, Medical Minded, Protz
- Product Description:
-
Product DescriptionHand Sanitizer
Company Announcement
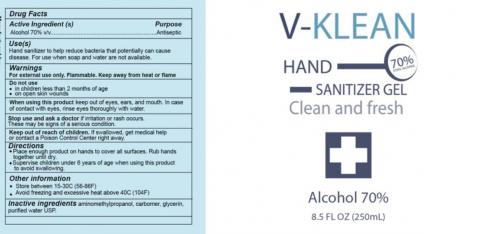
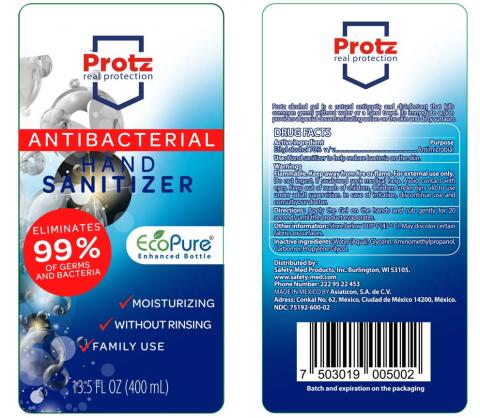
Mexico City, Mexico, Asiaticon SA de CV (Mexico) is voluntarily recalling all lots of V-Klean Hand Sanitizer Gel, Medically Minded Hand Sanitizer Gel and Protz Real Protection Antibacterial Hand Sanitizer sold in 13.5, 16.9 and 33.8 ounce bottles to the consumer level. The products are being recalled due to the potential presence of methanol (wood alcohol) and subpotent ethanol levels.
Risk Statement: Methanol has inferior antiseptic properties compared to ethanol. Substantial methanol exposure can result in nausea, vomiting, headache, blurred vision, permanent blindness, seizures, coma, permanent damage to the nervous system or death. Although all person using these products on their hands are at risk, young children who accidently ingest these products and adolescents and adults who drink these products as an alcohol (ethanol) substitute, are most at risk for methanol poisoning. To date Asiaticon SA de CV has not received reports of adverse events related to this recall.
The product is used as a hand sanitizer and is packaged in plastic clear bottles with clear tops with UPC Code:
V-Klean in 8.5 fl. oz. (250 ml) bottles: 716053704993
V-Klean in 16.9 fl. oz. (500 ml) bottles: 716053704993
V-Klean in 33.8 fl. oz. (1000 ml) bottles: 716053704993
Medically Minded in 16.9 fl. oz. (500 ml) bottles: 676753003782
Protz in 13.5 fl. oz. (400 ml) bottles : 7503019005002
The recall includes all lots. This product was exported to different distributors nationwide.
Asiaticon SA de CV is notifying its distributors by voluntary recall letter and consumers via this press release. Consumers that have the product subject to this recall should stop using and either contact Asiaticon SA de CV per the below for disposal instructions or return it to the place of purchase.
Consumers with questions regarding this recall can contact Asiaticon SA de CV at direccion@asiatic-connection.com (0052 1 55 21553488). In the US: at 929 394 3020 (available Monday to Friday 9.30 am 6 pm eastern time) Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to using this product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Link to CDER’s press statement: FDA updates on hand sanitizers consumers should not use
Company Contact Information
- Consumers:
- Asiaticon SA de CV
- 0052155 21553488, In the US: at 929 394 3020
- direccion@asiatic-connection.com