FDA Blood Supply and Demand Simulation Model Could Help Nation Prepare for Emergencies
May 27, 2018
By: Arianna Simonetti, Ph.D., and Richard Forshee, Ph.D.

Arianna Simonetti, Ph.D., is a Mathematical Statistician at FDA’s Office of Surveillance & Biometrics, Division of Biostatistics in the Center for Devices and Radiological Health.
Keeping the nation’s blood supply and demand system working efficiently can be a matter of life and death. That often means moving blood to meet critical needs when an area of the country experiences shortages. In fact, ensuring that blood gets to where it is needed, when it is needed, during emergencies is an important part of national security preparedness, and part of FDA’s mission.
That’s why FDA developed a blood supply model that estimates the amount of blood available in the system during both routine conditions and emergencies. This model is designed to help public health officials effectively plan strategies that will minimize any disruption of the blood supply should blood collection efforts be reduced as a result of an emergency. The model has the advantage of being easily customized to explore various “what-if” scenarios, so it could assist in the development of sound regulatory policy and strategic planning for emergency preparedness and medical responses requiring blood transfusions.

Richard Forshee, Ph.D., is Associate Director for Research at FDA’s Office of Biostatistics and Epidemiology in the Center for Biologics Evaluation and Research.
The model estimates how much blood is available during two types of emergencies—a pandemic influenza outbreak and a mass casualty event caused by the detonation of an improvised nuclear device. Either scenario could threaten the blood supply in two ways—by reducing the number of people available to donate or by increasing the amount of blood needed to respond to such an emergency.
To our knowledge, this model is the first attempt to estimate how much blood would be available for the U.S. blood system during potential national emergencies.
One of the challenges in dealing with an emergency is that red blood cells (RBCs) have a relatively short shelf life. The model allows us to calculate the supply—and the potential for shortages—based on how long RBCs have been in storage.
An earlier model designed by our group provided overall national daily estimates of the number of RBC units available in the demand system. Our new, inter-regional model divides the U.S. blood supply into four regions: East, West, South, and Midwest. This gives us a more fine-tuned look at the blood supply system’s response to emergencies by tracking the collection and transfer of blood across the different regions. It can also give us a snapshot of the blood supply at any time and in any region. This suggests that the model could help in planning for emergencies that trigger higher demands of blood in potentially affected regions.
Blood Availability:
A Model of Supply and Demand
The amount of blood that is collected and used in different regions of the country varies. The FDA model of the U.S. blood supply enables public health officials to estimate the availability of blood in each region at any given time. This helps minimize disruption and avoid shortages in the blood supply.
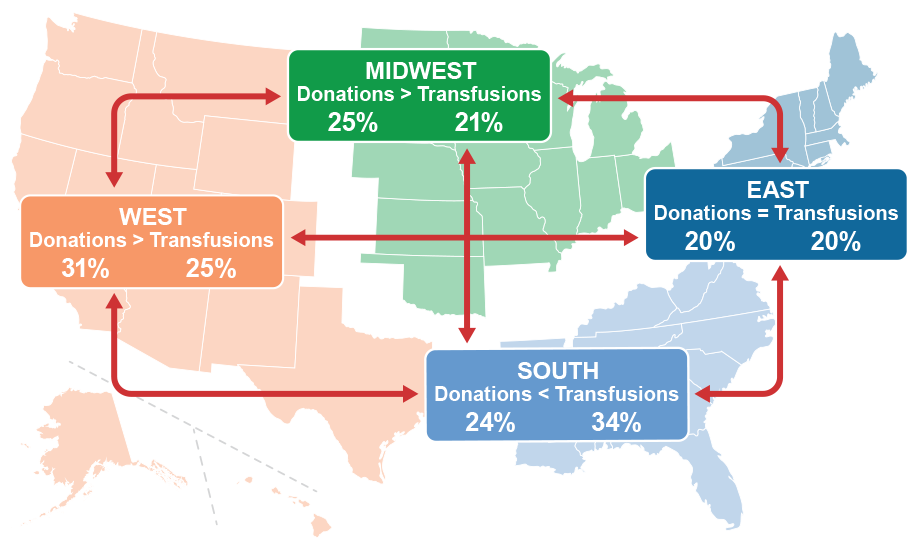
In the South, as shown in the example above, the amount of blood donated in a region may be less than the amount needed for transfusions in that region. This potential shortage in donations can be mitigated by blood transfers from other regions, as indicated by the arrows. The FDA model accounts for blood transfers among regions based on data provided by America’s Blood Centers, which collects about 55% of the U.S. blood supply.
To build a model that reflected actual blood collection and use in the four regions as closely as possible, we went to real-world sources of data. For example, we used the daily report on the national blood supply produced by America’s Blood Centers![]() to determine how much blood was available in each region. To track blood use in each region, we calculated regional daily RBC units transfused from the 2007-2012 Center for Medicare and Medicaid Center for Medicare and Medicaid Services database. We also used national estimates of blood collections and use from the 2011 National Blood Collection and Utilization Survey. Using this information, the model estimates the average number of RBC units available each day for each region.
to determine how much blood was available in each region. To track blood use in each region, we calculated regional daily RBC units transfused from the 2007-2012 Center for Medicare and Medicaid Center for Medicare and Medicaid Services database. We also used national estimates of blood collections and use from the 2011 National Blood Collection and Utilization Survey. Using this information, the model estimates the average number of RBC units available each day for each region.
To create our scenario for a pandemic, we used data on the outbreak of Pandemic A(H1N1) influenza from the Centers for Disease Control and Prevention and the weekly flu-like activity levels reported by the states.
Guided by a previously-developed computer model simulating the effect of a pandemic on blood donations and blood supplies in Germany, we ran a simulation on how the pandemic could affect the inter-regional supplies of blood in the U.S. One important finding was that the new model estimated that 541,000 RBC units were lost overall and that the South region had the highest percentage of blood lost (15.5%), while the East region had the lowest lost (13.8%), compared to the levels at the beginning of the pandemic.
For our simulation of the demand for RBC units needed following a mass casualty event caused by an improvised nuclear device, we used data on the expected casualties from each type of injury from a previous study. Our own inter-regional simulation let us predict the effect of such an event on the U.S. blood supply. For example, we saw that if the event occurred in the East region, given the current data available, this area of the country rapidly recovered to its original level of blood supply due to increased blood transfers from other regions.
The FDA model showed that, based on current levels of blood collection, use, and other factors, the U.S. blood supply and demand system is flexible and reliable enough to respond to these events.
But our simulation model is just an attempt to replicate the workings of the U.S. blood supply and demand system under various circumstances. Our conclusions could change if patterns of blood donation and use change. However, our experience with this model thus far shows that, as we add more accurate and current data and, make it available to planners, it could help the nation prepare for disruptions of blood collection and demand.
Arianna Simonetti, Ph.D., is a Mathematical Statistician at FDA’s Office of Surveillance & Biometrics, Division of Biostatistics in the Center for Devices and Radiological Health
Richard Forshee, Ph.D., is Associate Director for Research at FDA’s Office of Biostatistics and Epidemiology in the Center for Biologics Evaluation and Research
