Rapid Response Teams Mark 10 Years of Collaboration on Public Health Emergencies
By: Barbara Cassens, Director, Office of Partnerships, Office of Regulatory Affairs
Responding to health emergencies, such as the current multi-state outbreak of Salmonella Carrau linked to pre-cut melons, has long been a top priority at the U.S. Food and Drug Administration (FDA). The agency is working with the Centers for Disease Control and Prevention and our state partners to trace the specific source of these melons and collect samples for laboratory analysis. The outbreak required multiple agencies to work together to inspect and investigate. Such an effort was also required to trace the 2018 E. coli outbreak linked to romaine lettuce from California.
Marketplace globalization has made emergency response operations more complex, and as a result, the FDA relies on collaborative relationships with local, state, and federal agencies to successfully carry out our mission of protecting public health.
In 2008, to enable faster, more efficient responses to emergencies, the FDA launched a network of state-based Rapid Response Teams (RRT), comprised of multi-agency, multi-disciplinary teams that operate by the principles of the Incident Command System/National Incident Management System to respond to human and animal food emergencies. In an emergency, the Rapid Response Teams coordinate efforts to align the response activities of agencies that may have overlapping jurisdiction to prevent harm to consumers as quickly as possible.
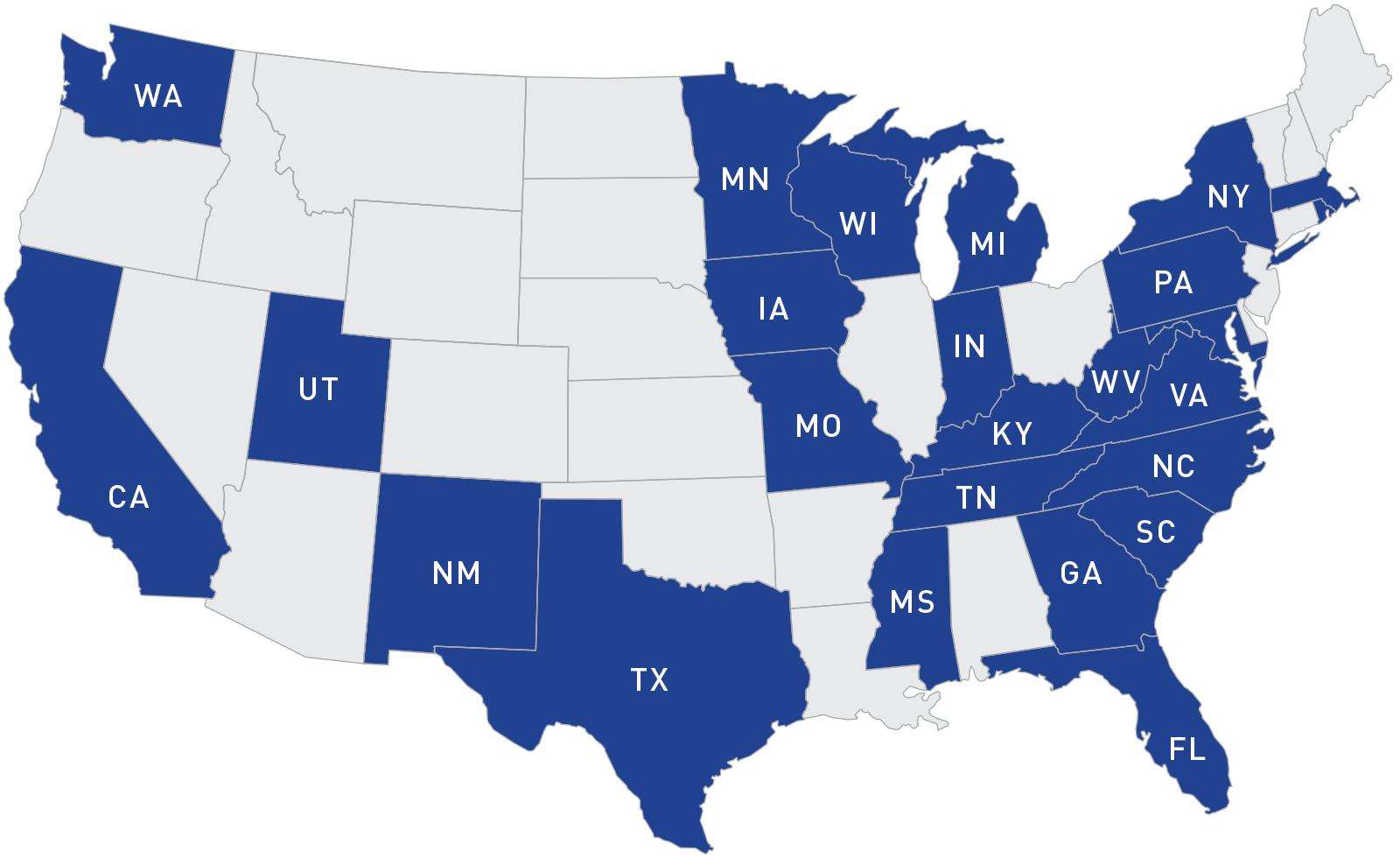
States Participating in the Rapid Response Team (RRT) Program from 2008 - 2018
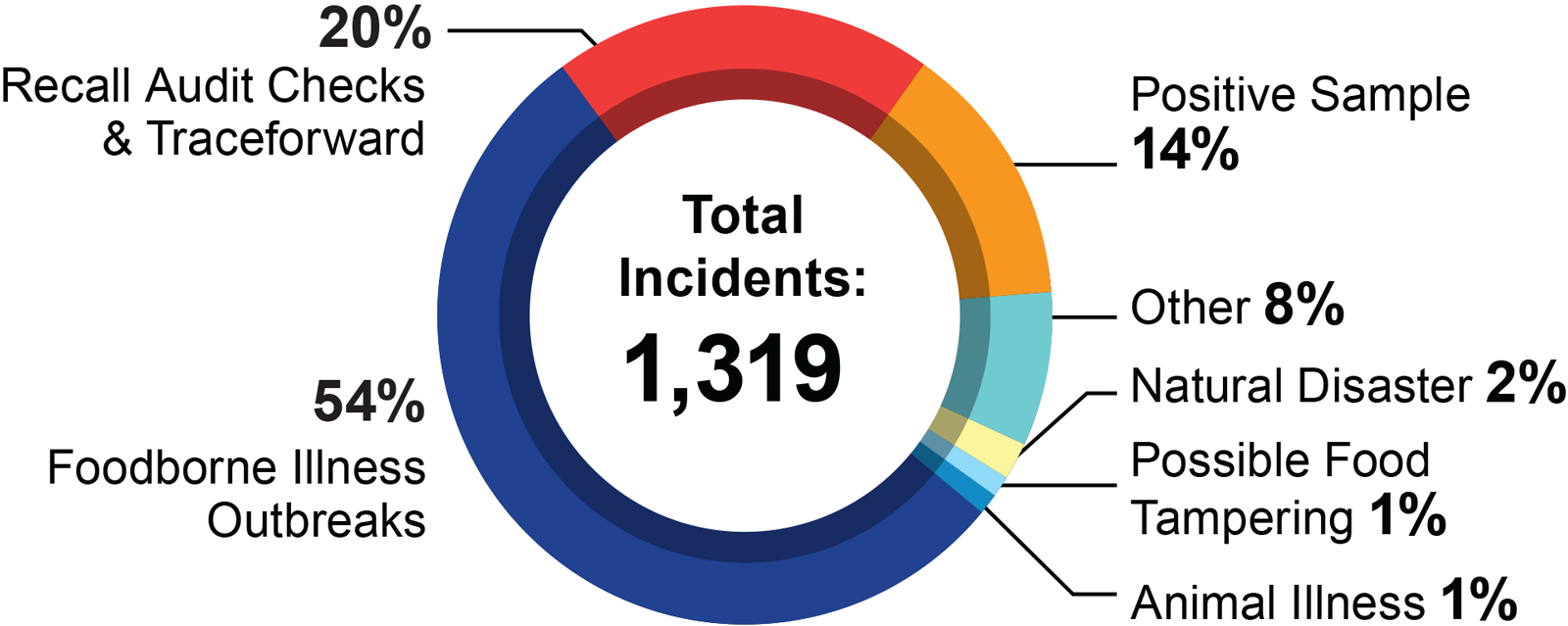
RRT Investigations by the Numbers
Types of RRT Investigations from September 2013 - August 2017
Infographics by the FDA’s Center for Food Safety and Applied Nutrition.
These teams have become valued partners in responding to outbreaks from contaminated human and animal foods, conducting large-scale recalls, and ensuring availability of safe foods during a natural disaster. The FDA can respond more rapidly during an emergency by leveraging the relationships and resources with local, state, and federal partners for the common public health goals we share.
For example, the FDA and the Rapid Response Teams from Maryland, California, Michigan, New York, and Georgia, as well as other federal, state, and local partners worked together to respond quickly and effectively to the 2018 romaine lettuce E coli outbreak. This outbreak resulted in 62 reported illnesses, 25 hospitalizations, and two life-threatening complications across 16 states and the District of Columbia. Through the Rapid Response Teams, the FDA and our state partners worked diligently to trace the contaminated lettuce to its source. This meant analyzing lettuce samples and environmental samples from produce farms. This work allowed the agency to narrow down the possible sources and issue timely consumer advisories to prevent further illness.
Today, 21 states have FDA-funded Rapid Response Teams, and three additional states participate in a voluntary capacity.
Federal, State and Local Collaboration: A Best-Teams Approach
Relationships and collaboration are immensely valuable for responding rapidly to an emergency. The Rapid Response Team model facilitates the relationships the FDA relies on in an increasingly complex marketplace. Each state has a unique organizational structure for regulatory and public health agencies that play a role in emergency response. A Rapid Response Team within a state can draw in needed partners from cities or counties within the state or from other federal agencies. It can also help agencies leverage their collective investigative resources to enhance efficiency.
Seventy-five percent of Rapid Response Team states have decentralized authority (based on Rapid Response Team program metrics for the 2016-17 grant year), meaning that local health departments have some jurisdiction over retail food service establishments within the state. States typically place regulatory and licensing authorities for human and animal food commodities in various state-level agencies, such as the Department of Health, Department of Agriculture, Department of Environment, Office of the State Chemist, or Board of Animal Health. For Rapid Response Teams, the challenge is in the concerted effort it takes to develop a common vision, approach, and operating plan for coordinating investigations with multiple partners playing distinct and related roles.
In addition, relationships between states are extremely important. Due to the complexities of our food production system, very few food products go from farm-to-fork within a single state. As a result, outbreaks rarely limit themselves to state lines, and a single outbreak may impact consumers from multiple states requiring multiple state partners to respond. Responders need to investigate many components of an outbreak: human illness and likely points of exposure, as well as the food production process from growing to harvesting, packing, re-packing, and processing. When an outbreak or other incident quickly jumps across state borders, pre-established relationships between Rapid Response Teams facilitate the rapid transfer of background information on the incident. Then, additional Rapid Response Teams can take quick action to move the investigation forward. During multi-state outbreaks linked to FDA-regulated products, Rapid Response Teams work closely with the FDA Coordinated Outbreak Response and Evaluation Network (CORE) Response Teams to ensure the Rapid Response Teams’ investigation activities are aligned with the national response strategy.
Advancing the work of Rapid Response Teams
2018 brought about change for the Rapid Response Team cooperative agreement, which is now part of the Flexible Funding Model, a comprehensive funding vehicle to support state programs for regulating manufactured food. This shift demonstrates the FDA’s commitment to supporting state food regulatory program infrastructure and enhanced capacity for integrated, rapid response. It is important to emphasize that Rapid Response Teams are a multi-agency partnership to address all hazards response across the farm-to-fork continuum. The Rapid Response Team activities speak to this, highlighting response and preparedness work for chemical, microbiological, and radiological contamination of human and animal food products, at retail, processing, and growing operations.
As a further testament to the importance of partnership and collaboration, in 2017, the FDA and Rapid Response Team states worked together to compile a best practices manual, which provides a set of concepts, definitions, tools, and examples organizations can use to develop core emergency response capabilities. Working together has proven to be the foundation and future of the Rapid Response Team program.
As an essential part of the national Integrated Food Safety System, Rapid Response Teams have paved the way as a best-teams approach to effective coordination and collaboration in a regulatory environment. Collectively, Rapid Response Teams are neighboring states, cooperating agencies, and at our core, public health protectors. As the FDA continues to address the challenges of globalization, we look forward to continued collaboration with our partners to further strengthen and diversify the Rapid Response Teams program in the decades to come.