Are There "FDA Registered" or "FDA Certified" Medical Devices? How Do I Know What Is FDA Approved?
You may have seen words like this on a website selling a medical device in the United States, sometimes even with an FDA logo:
- FDA Registered
- FDA Certified
- FDA Registration Certificate
Such words may be used to mislead you. Is that the same thing as FDA approved, FDA cleared, or FDA authorized? The short answer is NO. Here's why.
FDA Registration
Owners or operators of places of business (also called establishments or facilities) that are involved in the production and distribution of medical devices intended for use in the United States are generally required to register annually with the FDA.
It's important to understand:
For more details:
- For information on medical device establishment registration, see How to Study and Market Your Device > Device Registration and Listing.
- For information on FDA establishment registration in general, see FDA Basics for Industry > Registration and Listing.
Are there FDA Certificates?
When a business involved in the production and distribution of medical devices intended for use in the United States registers with the FDA, they do not receive a certificate from the FDA.
It's important to understand:
In addition, the FDA does not "certify" registration information for businesses that have registered and listed.
Misleading FDA Registration Certificates
Some firms sell medical devices in the United States alongside "FDA registration certificates," such as the sample certificate depicted here.
These certificates often have the look of an official government document and may include the FDA logo. However, FDA does not issue device registration certificates.
Firms that misleadingly display certificates alongside information about and photos of a device for sale in the United States to imply review or approval by FDA of the device misbrand the device in violation of the Federal Food, Drug, and Cosmetic Act.
Related information: FDA Calls on Certain Firms to Stop Producing and Issuing Misleading "FDA Registration Certificates"
To report suspected misuse of a registration certificate, please refer to Reporting Allegations of Regulatory Misconduct.
How Do You Know if the FDA Approved, Cleared, or Authorized a Medical Device?
The FDA provides several ways for you to check if the FDA approved or cleared a medical device or, as described below, if the FDA authorized the device to be used during a public health emergency.
Check for Approved and Cleared Products in the Devices@FDA Database: Devices@FDA is a catalog of approved and cleared medical device information from the FDA.
To search for FDA-approved or FDA-cleared products by device name or company name:
- Go to the Devices@FDA Database.
- In the Enter a search term in the space below field, type the name of the device or the company name. You can type the exact name of a specific device or a generic name for a category of devices (such as pacemaker).
- Click Search.
Example: Search for Pacemaker
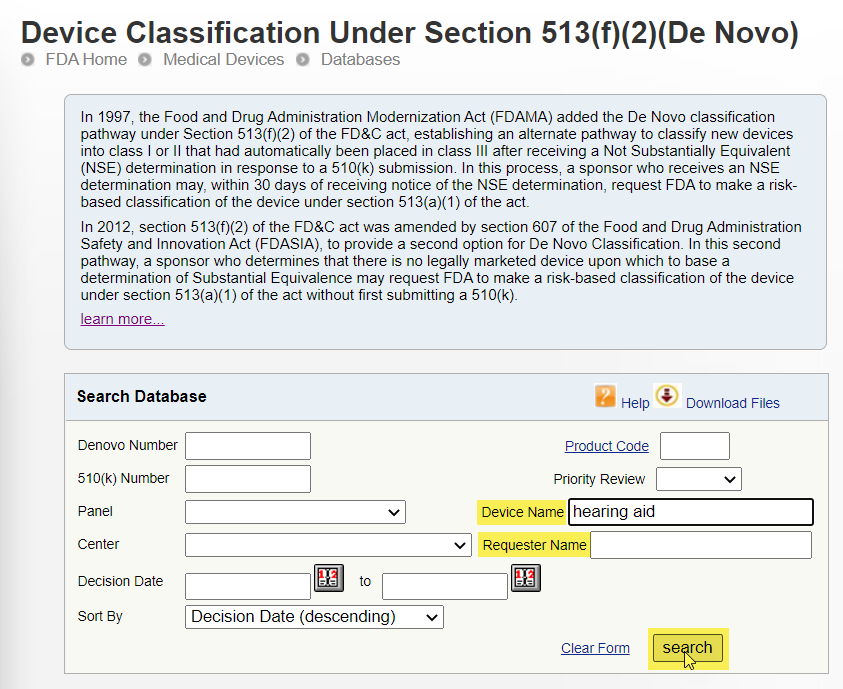
Check for Products in the De Novo Database: The FDA may review medical devices through the De Novo premarket review pathway, a regulatory pathway for low- to moderate-risk devices of a new type. A device reviewed through this pathway may be authorized for marketing in the United States. The term "De Novo" is Latin for "new."
To search for products by device name or company name:
- Go to the Device Classification Under Section 513(f)(2)(De Novo) database.
- Do one of the following:
- In the Device Name field, type the device name and click Search.
- In the Requester Name field, type the company name and click Search.
Example: Search for Hearing Aids
Check for Emergency Use Authorizations: In certain types of public health emergencies, such as during the COVID-19 pandemic, when the Secretary of Health and Human Services declares that circumstances exist justifying the authorization of emergency use of medical devices, the FDA may issue an Emergency Use Authorization (EUA) to authorize unapproved medical products or unapproved uses of approved medical products to be used in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions when certain criteria are met. This can help provide more timely access to critical medical devices that may help during the emergency when there are no adequate, approved, and available options.
To check medical device EUAs, go to Emergency Use Authorizations for Medical Devices.