CBER Common Entry Errors
Introduction
The Center for Biologics Evaluation and Research (CBER) regulates the following:
- Biological and related products including blood, vaccines, allergenics, tissues, and cellular and gene therapies. Biologics, in contrast to drugs that are chemically synthesized, are derived from living sources (such as humans, animals, and microorganisms), are not easily identified or characterized, and many are manufactured using biotechnology.
- Certain medical devices involved in the collection, processing, testing, screening, manufacture and administration of licensed blood, blood components, and tissue and cellular products
- HIV test kits used to screen donor blood, blood components, tissue and cellular products (HCT/Ps), and products used to diagnose, treat, and monitor persons with HIV and AIDS.
To expedite entry screening of CBER-regulated products by the Predictive Risk-based Evaluation for Dynamic Import Compliance Targeting (PREDICT) system, it is important that importers and entry filers provide accurate product codes, all relevant affirmations of compliance (AofC), and accurate identifiers for firms, in addition to the general entry data.
When complete and accurate entry data is supplied electronically, it can be used by the PREDICT system to “look-up” the information in the FDA’s databases, validate the information, and issue a system “May Proceed” if the line is not held due to any other screening criteria.
When the entry data provided is not complete and/or accurate, the “look-up” will fail and the entry line may be subject to delays in processing.
Details on applicable product codes and affirmations of compliance are included in the “Additional Information” section at the bottom of this page.
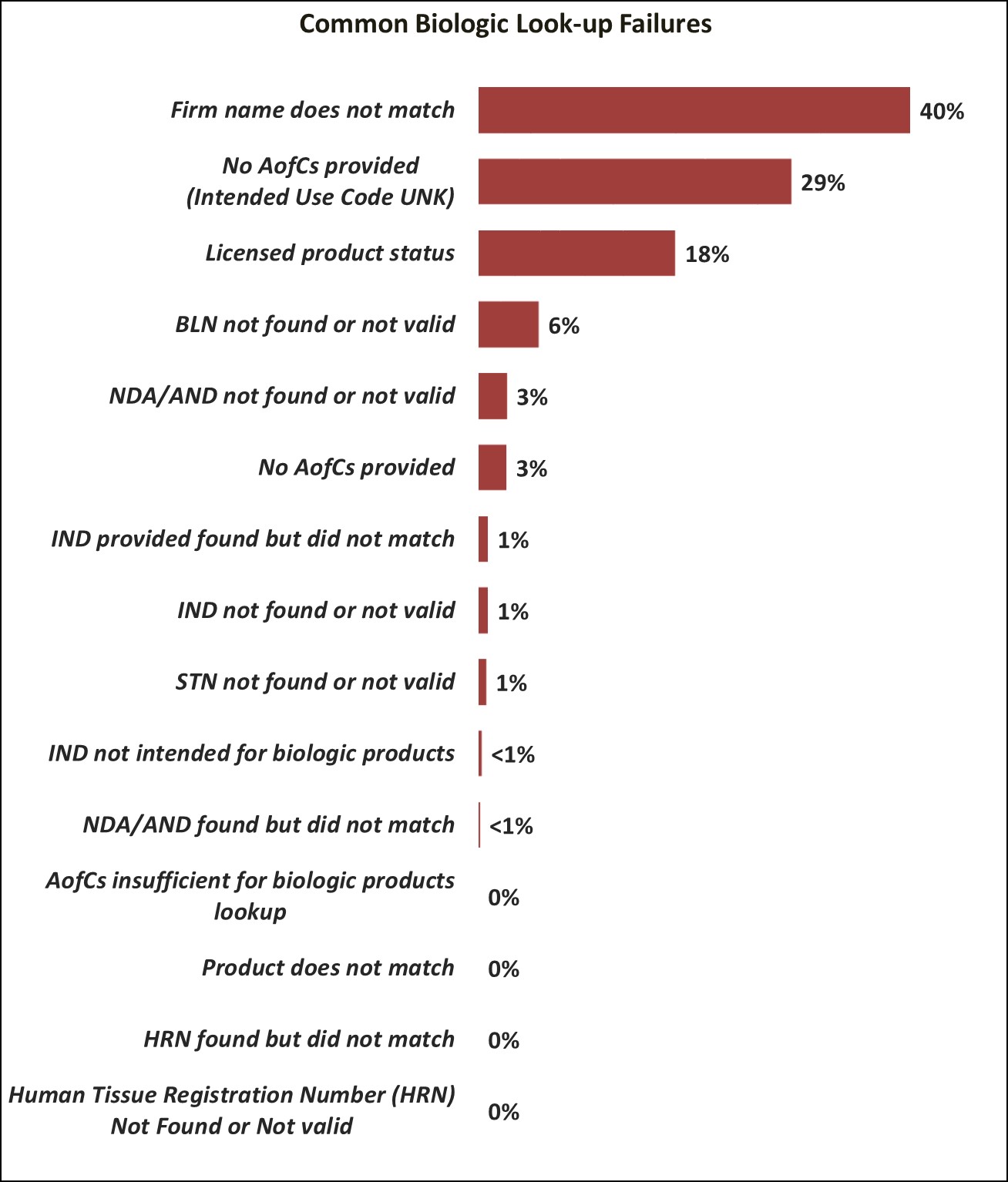
The following chart shows the most common look-up errors for CBER-regulated products during Fiscal Year 2023 (10/1/2022 to 9/30/2023).
When the entry data provided is not complete and/or accurate, the entry line may be subject to delays in processing. The table below provides additional information regarding these common errors to help you provide accurate and complete information to the FDA.
Common Biological Look-up Errors that Result in a Failed Look-up
| Error | Description | Additional Information |
|---|---|---|
|
No AofCs provided (Intended Use Code UNK) |
No Affirmations of Compliance (AofC) were provided for the entry and the intended use code was provided as (UNK). Submitting UNK as the Intended Use Code will subject the entry to manual review, which will cause delays. It might also make it difficult to identify which AofCs are mandatory and which are optional in the ACE FDA Supplemental Guide. If the product is FDA-regulated, supply the required AofC information and the appropriate intended use code. If the product is not FDA-regulated, and does not require declaration to the FDA, disclaim future entries. |
For more information see Affirmations of Compliance and the FDA Supplemental Guide. |
|
No AofCs provided |
No Affirmations of Compliance (AofC) were provided for this entry. If the product is FDA-regulated supply the required AofC information. If the product is not FDA-regulated, and does not require declaration to the FDA, disclaim future entries. |
For more information see Affirmations of Compliance. |
|
AofCs insufficient for biologic products lookup |
The product was declared as a biologic product (Industry code 57), however, the Affirmations of Compliance (AofC) provided were insufficient to perform the lookup for biologic products. Verify the product code and/or AofC code or qualifier. |
For more information see Importing CBER regulated products into the United States |
|
STN not found or not valid |
Submission Tracking Number (STN) transmitted could not be found in the FDA's biologic products database, is not valid, or was declared in an improper format. Verify correct AofC code and corresponding qualifier. |
The qualifier for this affirmation should be a six-digit submission tracking number. Information on the STN numbers can be found from the link below. Or the STN could have been transferred to CDER. A complete listing of STNs transferred to CDER is available from the link below. |
|
BLN not found or not valid |
The Biologics License Number (BLN) transmitted could not be found in the FDA's biologic products database, is not valid, or was declared in an improper format. Verify correct AofC code and corresponding qualifier. |
For more information see the list of licensed establishments and applicable products available |
|
Firm name does not match |
The firm name provided in the entry did not match the firm name on file for the AofC transmitted. Verify that the firms supplied on the entry match those filed with CBER. |
For more information see Biologics Products & Establishments |
|
Product does not match |
The product transmitted for the entry did not match the corresponding product for the AofC transmitted. Verify that the product claimed on the entry matches the product on file with CBER. |
For more information see Biologics Products & Establishments |
|
Licensed product status |
The product status for licensed biologic products must be "Approved." The product status of "Approved" was not found for the declared licensed biologic product. Verify that the licensed biologic product has been approved. |
|
|
IDE/IND provided/found but did not match |
The product status for investigational biologic products must be "Active" or "Exempt". Verify that the IND has the correct status. The IND may also be transferred to the Center for Drug Evaluation and Research |
The investigational biologic product in the line may be transferred to the Center for Devices and Radiological Health (IDE) or the Center for Drug Evaluation and Research (IND). For more information see INDs transferred to CDER |
|
IDE/IND not found or not valid |
The Investigational New drug (IND) or Investigational Device Exemption (IDE) transmitted could not be found in FDA's biologic products database, is not valid, or was declared in an improper format. Verify correct AofC code and corresponding qualifier. |
|
|
IND not intended for biologic products |
The product was declared as a biologic product (Industry code 57), however, the IND declared is insufficient to perform the biologic product lookup. Verify the Industry code and/or the AofC declared. If the IND has been transferred to CDER, use a product code that identifies the product as a drug, not a biologic. |
|
|
PMA/PMN/NDA/AND not found or not valid |
New Drug Application (NDA), Abbreviated New Drug Application (AND), Premarket Approval (PMA), or Premarket Notification number (510(K)) transmitted could not be found in the FDA's biologic product database, is not valid, or was declared in an improper format. Verify correct AofC code and corresponding qualifier. |
For more information see Biologics Products & Establishments |
|
PMA/PMN/NDA/AND found but did not match |
New Drug Application (NDA), Abbreviated New Drug Application (AND), Premarket Approval (PMA), or Premarket Notification number (510(K)) transmitted did not match entry data (firm and/or product) transmitted for the entry. Verify the firms and product listed on the line match what was submitted for the NDA, ANDA, PMA, or PMN. |
For more information see Biologics Products & Establishments |
|
Human Tissue Registration Number (HRN) Not Found or Not valid |
Human tissue registration number (HRN) transmitted could not be found in the FDA's biologic product database, was not valid, or was declared in an improper format. Verify correct AofC code and corresponding qualifier. |
Human Cell and Tissue Establishment Registration Public Query |
|
HRN found but did not match |
Human tissue registration number (HRN) transmitted did not match entry data (firm and/or product) transmitted for the entry. Verify the firms and product listed on the line match what was submitted to CBER for the HRN. |
Human Cell and Tissue Establishment Registration Public Query |