CFC-Free Medication for an Ailing Ozone Layer
by Tamar Nordenberg
More than 20 million Americans, including those who use metered-dose inhalers for their asthma or chronic obstructive pulmonary disease, could be affected if the Food and Drug Administration finalizes a policy to phase out medical products that contain chlorofluorocarbons (CFCs).
FDA is seeking comments from the public on a rule the agency first proposed in March that also covers CFC-containing products far less common than metered-dose inhalers, such as nitroglycerin sprayed into the mouth to relieve chest pain.
Metered-dose inhalers and some other medical products use CFCs as propellants to carry the drug to the lungs or elsewhere in the body. While these products don't present a direct threat to users' health, they contain CFCs that eat away at the Earth's protective ozone layer and lead to increased ultraviolet radiation reaching the Earth's surface. Because increased UV rays are linked to skin cancer, cataracts, suppressed immune systems, and other health problems, CFCs indirectly can contribute to these conditions.
FDA's move toward phasing out CFC-containing medical products is part of a worldwide reduction in CFC production under the international agreement "Montreal Protocol on Substances that Deplete the Ozone Layer" and the U.S. Clean Air Act. (See "Planet-Wide Phaseout.") Signed by more than 160 countries, the protocol called for a general ban on CFC production in industrialized countries by January 1996.
The use of CFCs in essential medical devices, including metered-dose inhalers, has been exempt from the ban.
"While we have an international agreement which aims for zero CFC use, FDA will always keep in mind that millions of Americans depend on these products to breathe," says Tunde Otulana, M.D., a medical officer with FDA's division of pulmonary drug products and head of an agency CFC work group. "Our goal is to protect the environment without putting patients' health at risk."
Under the approach being considered, the sale of the remaining CFC-containing medical products would be phased out as safe and effective alternatives become available.
"Naturally, patients may have concerns because they will have to switch to new medications as the products they have trusted for many years are replaced," Otulana says. "But while the look of the non-CFC products may be different, and the taste may be different, FDA is working to ensure that the effectiveness and safety of the drugs will be comparable to patients' current medications."
Public Perspective
The agency published an "advance notice of proposed rulemaking" in the March 6, 1997, Federal Register with a 60-day public comment period ending May 5. The notice is available on the Government Printing Office's web site (http://www.access.gpo.gov/su_docs/aces/aaces002.html). Paper copies can be obtained through FDA's fax-on-demand system by calling (1-800) 342-2722 and requesting document number 0513.
Generally, the rule in its proposed form states that CFC-containing medical products would be considered for a phaseout once these conditions are met:
- Acceptable treatment alternatives exist for the particular metered-dose inhaler or other drug product so each patient can find a product that meets his or her medical needs.
- The alternatives are marketed for at least one year and are accepted by patients.
- The supply of alternative products is sufficient to ensure that there will be no drug shortages.
After considering the public's comments on the March notice and incorporating changes, FDA will publish a proposed rule in the Federal Register. The public will then have another 60 days to comment. Comments should be sent to FDA's Dockets Management Branch, HFA-305, Room 1-23, 12420 Parklawn Drive, Rockville, MD 20857. (See "Inside FDA: How to Comment on Proposals and Submit Petitions" in the April 1996 FDA Consumer.)
Products with Promise
Faced with the prospect of the eventual removal of CFC-containing medical products from the market, companies are developing alternative products, Otulana says.
FDA has already approved one: 3M Pharmaceuticals' Proventil HFA (albuterol sulfate), which is marketed by Key Pharmaceuticals Inc. FDA approved the drug in August 1996. Its active ingredient, albuterol, remains the same as the now-marketed CFC-containing Proventil metered-dose inhaler. But the propellant hydrofluoroalkane (HFA) carries the drug into the lungs with no known ozone-depleting chemicals.
"[I]n the very near future there will be a whole family of HFA-propelled products to replace the family of CFC inhalers," says Maria J. Westfall, global program manager at 3M.
Dry powder inhalers that can hold several doses of medication are another possible alternative to CFCs. The dry powder drug substance would be inhaled without using any propellant.
When patients switch to the new technologies, they will understandably have concerns, says Nancy Sander, president of the patient advocacy organization Mothers of Asthmatics. The key to a successful transition, she says, is to educate health-care providers and patients about the therapeutic, as well as environmental, benefits of the switch to the new medicines.
New technologies can have direct benefits for patients, she says. "[Many] companies are investigating not only replacing CFC propellants but also building a better inhaler. They could get down to pure drug in some cases, with no additive whatsoever. Then you'd be getting more of what you need and less of what you don't."
Most patients probably won't hesitate to switch to the new medical products, according to a 1996 American Lung Association study. About 90 percent of patients surveyed said they would switch to a non-CFC inhaler under a doctor's recommendation.
And patients' confidence is well-placed, Otulana says: "The policy we are proposing will not jeopardize the availability of safe, effective medical treatments."
Tamar Nordenberg is a staff writer for FDA Consumer.
Planet-Wide Phaseout
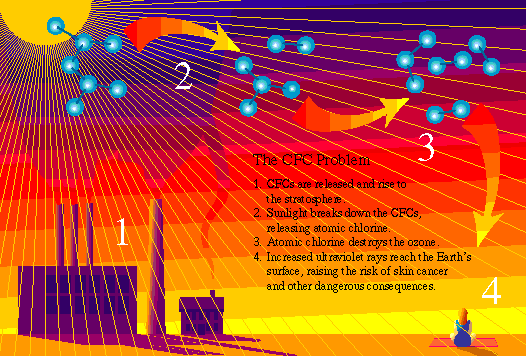
CFCs (chlorofluorocarbons)--made up of fluorine, chlorine and carbon--have been widely used in industry for decades because of the chemicals' stability, nonflammability, and low toxicity. "The fact that [CFCs] don't break down is a benefit for their many applications but a curse for the stratospheric ozone," says Tom Land, environmental protection specialist with the U.S. Environmental Protection Agency.
Because CFCs are not dissolved by rain, over the years they rise to the ozone layer--in the stratosphere, about 10 to 20 miles (15 to 30 kilometers) above the Earth's surface. There, CFCs can linger for 100 years, while the sun's harsh radiation breaks them down, releasing atomic chlorine. One chlorine atom can destroy more than 100,000 ozone molecules.
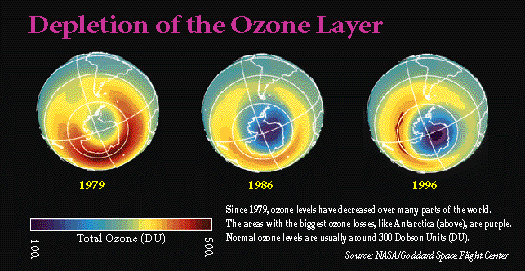
Scientists have discovered that an ozone "hole" has developed over Antarctica. The ozone levels there sometimes drop by up to 60 percent, depending on the season, the weather, and other environmental factors. Over the United States, as well as the rest of North America, Europe, and Australia, ozone levels have fallen 5 to 10 percent since the 1960s, according to EPA.
Damage to the ozone layer has dangerous consequences for humans because the ozone molecules function as a shield from the sun's radiation, absorbing some of the harmful ultraviolet radiation before it reaches the Earth's surface.
Skin cancer is one of the most serious dangers from ozone depletion. A United Nations study estimated that a 1 percent decrease in the atmospheric ozone concentration translates into a 2 percent increase in the rate of non-melanoma skin cancer, which can be fatal.
"If we hadn't done anything about CFCs, the depletion of the ozone would have gotten worse, and we would have had to significantly change our lifestyle," Land says. "We wouldn't be able to go outside without wearing hats all the time, and we wouldn't be able to build things out of plastic and rubber that would be exposed to sunlight because they would deteriorate much faster."
The first move to cut back on CFCs in the United States came in the 1970s, when EPA banned use of the chemicals as aerosol propellants. But production of CFCs grew rapidly with the discovery that they could be used in other ways, mainly as refrigerants for air-conditioning homes and cars, solvents for cleaning electronic equipment and precision parts, and foam blowing agents for making foam products, such as food service packaging.
Scientific evidence of the dangerous, rapid depletion of stratospheric ozone led to a United Nations-sponsored international agreement, the Montreal Protocol on Substances that Deplete the Ozone Layer. The agreement calls for the phaseout of CFCs and other ozone-destroying chemicals. Its goal was to virtually end CFC production in the United States and other industrialized countries by January 1996. The timetable allows developing countries an extra 10 years to stop making CFCs.
"Some time in the middle of the next century, the ozone layer may recover to what is considered a normal level, as measured back in the 1960s," Land says.
As a result of worldwide efforts to protect the ozone layer, EPA expects 295 million fewer cases worldwide of non-melanoma skin cancer over the next century.
To learn more about CFCs and the environment, call EPA's toll-free Ozone Protection Hotline at (1-800) 296-1996, or visit the agency's web site at http://www.epa.gov/ozone/.
--T.N.