Use of Cyclin-Dependent Kinase 4/6 Inhibitor with Hormonal Therapy in Metastatic Breast Cancer—Efficacy in Patient Subgroups
CDER researchers analyzed data from patients with hormone receptor–positive, human epidermal growth factor receptor 2-negative advanced or metastatic breast cancer to determine whether any patient or tumor characteristics were associated with more (or less) benefit from adding a cyclin-dependent kinase inhibitor to a hormonal agent. All patient subgroups benefited from the addition of a cyclin-dependent kinase inhibitor to hormonal therapy.
The Scientific Challenge
Cyclin-dependent kinases (CDKs) are enzymes that play an important role in cell division, making them attractive therapeutic targets for certain cancers, including some types of breast cancer. CDK 4/6 inhibitors in combination with hormonal therapy (aromatase inhibitor or fulvestrant) are FDA-approved for the first- or second-line treatment of patients with hormone receptor–positive, human epidermal growth factor receptor 2 (HER2)–negative advanced or metastatic breast cancer.
In clinical trials, patients may not benefit equally from the same treatments. Differences in treatment efficacy may be due to patient characteristics, such as age and race, or to characteristics of their tumors, such as whether one or both hormone receptors are present or whether the cancer had already metastasized at the time of first diagnosis. The number of patients in various subgroups, in which patients are grouped according to a set of characteristics, or factors, is often limited in a single trial. By grouping patients who share one or more of these characteristics across many trials, however, it is possible to explore whether one or more of these factors may be associated with different degrees of benefit from treatment.
Attempt to Identify Patient or Tumor Characteristics that Impact Efficacy of CDK Inhibitors in Patients with Breast Cancer
CDER researchers investigated whether adding a CDK 4/6 inhibitor to a hormonal agent improves the duration of progression-free survival (PFS) in certain subgroups of patients with hormone receptor–positive, HER2-negative advanced or metastatic breast cancer. They pooled data from seven breast cancer clinical trials submitted to FDA in support of drug marketing applications that were approved before January 1, 2019. Taken together, the seven breast cancer trials included a total of 4,200 patients and allowed for a larger patient sample size for each of the subgroups analyzed, compared to the sample size for any single trial alone. In each trial, the participants were randomly assigned to receive a hormonal agent (an aromatase inhibitor [letrozole or anastrozole] or fulvestrant) together with either a CDK 4/6 inhibitor or placebo.
Subgroups were chosen based on prior data about characteristics associated with likelihood of response to hormonal therapy in patients with breast cancer. CDER researchers analyzed the following subgroups in their pooled analysis: age ≤ 40, progesterone receptor negativity, a shorter time interval from disease diagnosis until recurrence (i.e., disease-free interval), metastatic disease at the time of diagnosis, and metastases involving an internal organ such as the liver or lung, all of which are considered to be associated with biologically more aggressive tumors and decreased sensitivity to therapy with hormonal agents. By contrast, breast cancer of lobular histology and breast cancer that has metastasized only to bone may be biologically less aggressive and respond well to treatment with a hormonal agent alone. Table 1 indicates the subgroups of patients with breast cancer pooled from the seven trials for exploratory analysis.
Findings
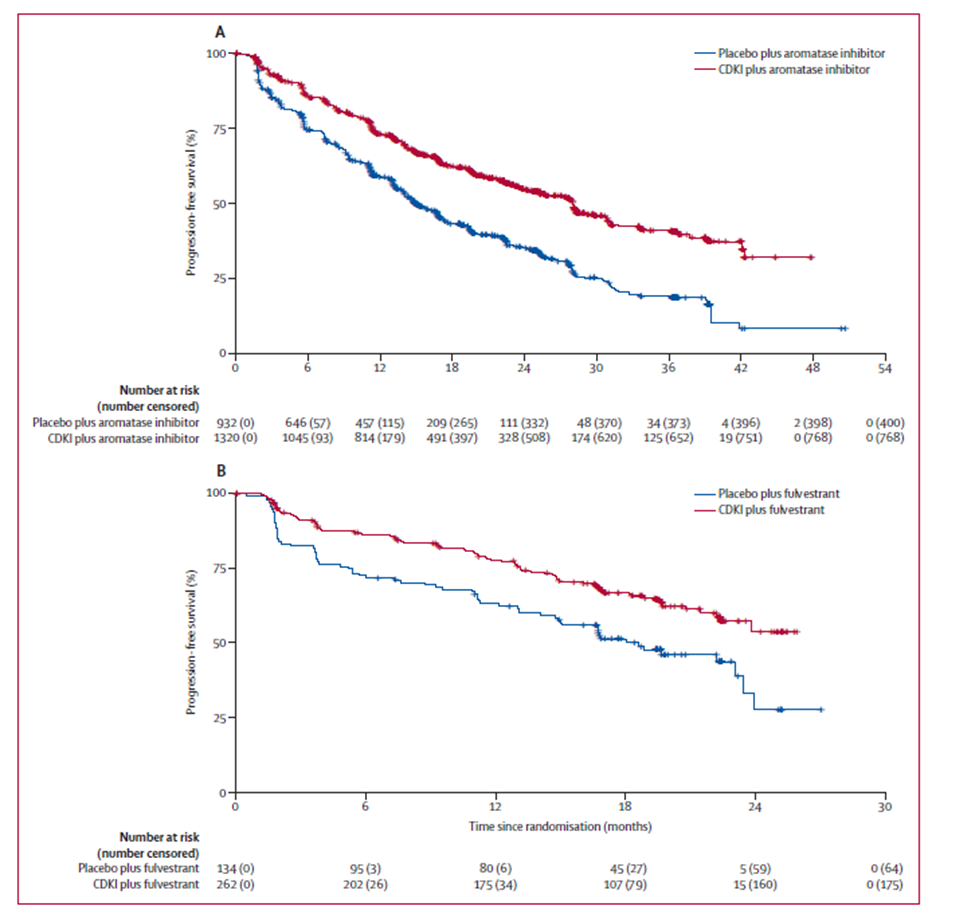
The addition of a CDK 4/6 inhibitor to an aromatase inhibitor as the first-line therapy increased the median PFS by 13.1 months (range 13.0-13.3 months in individual trials, 1 trial median not estimable) (Table 1). The addition of a CDK 4/6 inhibitor to fulvestrant, irrespective of the line of therapy, increased the median PFS by 7.4 months (range 6.8-7.8 months in individual trials) (Table 1). Indeed, PFS increased irrespective of whether the hormonal agent was an aromatase inhibitor or fulvestrant (Figure 1). Importantly, an increase in median PFS was observed in all clinicopathological subgroups (Table 2) as well as across age and ethnicity subgroups (Table 3). Only results in the White and Asian ethnicity subgroups are reported, owing to the small number of patients of other races or ethnicities who enrolled in the seven trials.
Based on the results of the pooled analysis, adding a CDK 4/6 inhibitor to a hormonal agent appeared to show a consistent PFS benefit for all subgroups analyzed. This finding will inform clinical decision-making regarding the risks and benefits of administering a CDK 4/6 inhibitor in conjunction with a hormonal agent versus monotherapy with a hormonal agent and reinforces that all patients with hormone receptor–positive, HER2-negative metastatic breast cancer should receive a CDK inhibitor along with hormonal therapy according to the FDA-approved indications.
Implications
CDER researchers analyzed several important subgroups of patients treated with modern hormone-based regimens. The benefit of adding a CDK 4/6 inhibitor to a hormonal agent was consistent across all subgroups, which provides important data to clinicians and patients for treatment decision-making. This study will also inform the design and analysis of future clinical trials of hormone receptor–positive, HER2-negative metastatic breast cancer.
Future Directions
The CDER researchers recommend further study of tissue biomarkers that may predict response or resistance to CDK 4/6 inhibitors. Such investigations would enable identification of patients likely to have a favorable outcome with a hormonal agent alone, as well as identification of those patients likely not to benefit from existing combinations and for whom participation in clinical trials of novel agents should be recommended.
Reference
Gao JJ, Cheng J, Bloomquist E, et al. CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: a US Food and Drug Administration pooled analysis. Lancet Oncol. 2020;21(2):250-260. doi:10.1016/S1470-2045(19)30804-6.
Gao JJ, Cheng, J, Beaver, JA, Prowell, TM. Ethnicity-based differences in breast cancer features and responsiveness to CDK4/6 inhibitors combined with endocrine therapy – Authors’ reply. Lancet Oncol. 2020;21(3):e131. doi: 10.1016/S1470-2045(20)30095-4.
How can this work improve the treatment of certain patients with breast cancer?
The consistent benefit for patients with breast cancer of adding a CDK 4/6 inhibitor to hormonal therapy will inform clinical practice, patient counseling, and design of future clinical trials.
Table 1. Progression Free Survival Across All Pooled Studies
|
Patients, n |
Events, n/Patients, N (%) |
Hazard Ratio1 |
Median PFS (95% CI2), Months |
Difference in Estimated Median PFS, Months |
|||||
|---|---|---|---|---|---|---|---|---|---|
|
CDKI + Hormone |
Placebo + Hormone |
Overall (95% CI) |
Range in Individual Trials |
CDKI + Hormone |
Placebo + Hormone |
Overall |
Range in Individual Trials |
||
|
All pooled trials |
4200 |
1192/2616 (46%) |
982/1584 (62%) |
0.59 (0.54‑0.64) |
0.50‑0.61 |
– |
– |
8.8 |
6.8‑13.3 MNE in one trial |
|
Aromatase inhibitor (first‑line setting) |
2252 |
552/1320 (42%) |
532/932 (57%) |
0.55 (0.49‑0.62) |
0.54‑0.56 |
28.0 (25.3‑29.1) |
14.9 (14.0‑16.7) |
13.0 |
13.0‑13.3 MNE in one trial |
|
All Fulvestrant trials |
1948 |
640/1296 (49%) |
450/652 (69%) |
0.58 (0.51‑0.65) |
0.50‑0.61 |
– |
– |
7.4 |
6.8‑7.8 |
|
Fulvestrant (first‑line setting) |
396 |
87/262 (33%) |
70/134 (52%) |
0.58 (0.42‑0.80) |
0.52‑0.59 |
NE (22.4‑NE) |
18.6 (14.8‑23.5) |
NE |
MNE in two trials |
|
Fulvestrant (second-line and beyond setting |
1552 |
553/1034 (53%) |
380/518 (73%) |
0.56 (0.49‑0.64) |
0.50‑0.61 |
– |
– |
6.9 |
5.5‑7.3 |
Abbreviations: CDKI, cyclin-dependent kinase inhibitor; CI, confidence interval; MNE, median not estimable; NE, not estimable; PFS, progression free survival
1 Estimated by a Cox regression model with treatment as the only covariate.
2 Kaplan–Meier estimate, reported only for pooled trials in the first-line setting.
Table 2. Efficacy Across Clinicopathological Subgroups
|
Subgroup |
Patients, n |
Events, n/Patients, N (%) |
Hazard Ratio1 |
Median2 PFS (95% CI3), Months |
Difference in Estimated Median PFS, Months |
||||
|---|---|---|---|---|---|---|---|---|---|
|
CDKI + Hormone |
Placebo + Hormone |
Overall (95% CI) |
Range in Individual Trials |
CDKI + Hormone |
Placebo + Hormone |
Overall |
Range in Individual Trials |
||
|
Progesterone receptor negative |
|||||||||
|
Overall |
740 |
229/459 (50%) |
200/281 (71%) |
0.51 (0.43‑0.62) |
0.40‑0.57 |
– |
– |
9.2 |
7.3‑18.1; median NE in one trial |
|
Aromatase inhibitor trials |
273 |
62/155 (40%) |
81/118 (69%) |
0.40 (0.29‑0.56) |
0.40‑0.42 |
27.5 (18.2‑29.5) |
9.4 (5.6‑13.0) |
18.1 |
8.5‑18.1; median NE in one trial |
|
Fulvestrant trials4 |
467 |
167/304 (55%) |
119/163 (73%) |
0.56 (0.44‑0.71) |
0.51‑0.57 |
– |
– |
7.0 |
7.3‑9.8 |
|
Progesterone receptor positive |
|||||||||
|
Overall |
2719 |
697/1667 (42%) |
599/1052 (57%) |
0.64 (0.57‑0.71) |
0.49‑0.64 |
– |
– |
7.9 |
6.2‑12.6; median NE in two trials |
|
Aromatase inhibitor trials |
1295 |
243/710 (34%) |
288/585 (49%) |
0.59 (0.50‑0.70) |
0.56‑0.60 |
29.1 (26.2‑NE) |
16.6 (14.8‑19.4) |
12.5 |
12.6 in one trial; median NE in two trials |
|
Fulvestrant trials4 |
1424 |
454/957 (47%) |
311/467 (67%) |
0.60 (0.52‑0.69) |
0.49‑0.64 |
– |
– |
5.8 |
6.2‑8.3 |
|
Disease free ≤ 12 months |
|||||||||
|
Aromatase inhibitor trials |
180 |
70/114 (61%) |
48/66 (73%) |
0.58 (0.40‑0.84) |
0.45‑1.63 |
16.9 (14.1‑24.6) |
11.1 (8.3‑16.7) |
5.8 |
0.8‑5.9; median NE in two trials |
|
Disease free > 12 months |
|||||||||
|
Aromatase inhibitor trials |
1181 |
283/675 (42%) |
292/506 (58%) |
0.55 (0.47‑0.65) |
0.52‑0.61 |
27.5 (22.6‑29.5) |
14.0 (13.0‑16.5) |
13.5 |
5.8‑14.3 |
|
Metastatic disease at diagnosis |
|||||||||
|
Overall |
1222 |
309/772 (40%) |
250/450 (56%) |
0.59 (0.50‑0.70) |
0.30‑0.72 |
– |
– |
11.6 |
5.6‑16.5; median NE in two trials |
|
Aromatase inhibitor trials |
752 |
168/450 (37%) |
156/302 (52%) |
0.56 (0.45‑0.69) |
0.30‑0.69 |
30.8 (27.5‑36.5) |
17.6 (14.9‑22.5) |
13.2 |
5.6‑16.5; median NE in two trials |
|
Fulvestrant trials4 |
470 |
141/322 (44%) |
94/148 (64%) |
0.58 (0.45‑0.76) |
0.37‑0.72 |
– |
– |
9.3 |
5.8‑11.8 |
|
No metastatic disease at diagnosis |
|||||||||
|
Overall |
2943 |
871/1820 (48%) |
722/1123 (64%) |
0.59 (0.53‑0.65) |
0.51‑0.63 |
– |
– |
8.1 |
6.1‑15.9 |
|
Aromatase inhibitor trials |
1495 |
381/866 (44%) |
375/629 (60%) |
0.55 (0.48‑0.64) |
0.51‑0.63 |
27.1 (22.6‑28.0) |
14.0 (12.9‑15.6) |
13.1 |
6.4‑15.9 |
|
Fulvestrant trials4 |
1448 |
490/954 (51%) |
347/494 (70%) |
0.58 (0.50‑0.66) |
0.54‑0.59 |
– |
– |
6.0 |
6.1‑8.3 |
|
Lobular histology5 |
|||||||||
|
Overall |
269 |
111/186 (60%) |
64/83 (77%) |
0.58 (0.43‑0.80) |
0.32‑1.07 |
– |
– |
6.9 |
−3.9‑12.0 |
|
Aromatase inhibitor trials |
144 |
58/97 (60%) |
36/47 (77%) |
0.60 (0.39‑0.91) |
0.48‑1.07 |
23.6 (16.1‑28.1) |
16.1 (10.8‑22.4) |
7.5 |
−3.9‑9.8 |
|
Fulvestrant trials4 |
125 |
53/89 (60%) |
28/36 (78%) |
0.43 (0.27‑0.70) |
0.32‑0.57 |
– |
– |
9.3 |
4.3‑12.0 |
|
Ductal histology |
|||||||||
|
Overall |
1488 |
499/988 (51%) |
357/500 (71%) |
0.53 (0.46‑0.61) |
0.45‑0.55 |
– |
– |
10.7 |
5.9‑15.3 |
|
Aromatase inhibitor trials |
778 |
248/513 (48%) |
186/265 (70%) |
0.51 (0.42‑0.62) |
0.45‑0.55 |
28.1 (24.3‑32.8) |
14.0 (11.2‑16.7) |
14.1 |
14.1‑15.3 |
|
Fulvestrant trials4 |
710 |
251/475 (53%) |
171/235 (73%) |
0.52 (0.43‑0.63) |
0.49‑0.49 |
– |
– |
6.7 |
5.9‑11.0 |
|
Bone-only metastases |
|||||||||
|
Overall |
946 |
228/594 (38%) |
199/352 (57%) |
0.54 (0.45‑0.66) |
0.38‑0.70 |
– |
– |
11.3 |
3.6‑25.3; MNE in two trials |
|
Aromatase inhibitor trials |
493 |
102/284 (36%) |
107/209 (51%) |
0.51 (0.38‑0.67) |
0.40‑0.70 |
33.6 (28.0‑NE) |
17.5 (16.2‑25.2) |
16.1 |
3.6‑25.3; MNE in two trials |
|
Fulvestrant trials4 |
453 |
126/310 (41%) |
92/143 (64%) |
0.53 (0.40‑0.69) |
0.38‑0.62 |
– |
– |
8.7 |
5.5‑10.3 |
|
Not bone-only metastases |
|||||||||
|
Overall |
3254 |
964/2022 (48%) |
783/1232 (64%) |
0.60 (0.54‑0.66) |
0.47‑0.68 |
– |
– |
8.2 |
6.4‑14.8; MNE in one trial |
|
Aromatase inhibitor trials |
1759 |
450/1036 (43%) |
425/723 (59%) |
0.56 (0.49‑0.64) |
0.50‑0.62 |
25.7 (22.8‑28.1) |
14.0 (13.1‑15.2) |
11.7 |
9.4‑14.8; MNE in one trial |
|
Fulvestrant trials4 |
1495 |
514/986 (52%) |
358/509 (70%) |
0.59 (0.52‑0.68) |
0.47‑0.68 |
– |
– |
7.1 |
6.4‑7.6 |
|
Liver or lung metastases |
|||||||||
|
Overall |
2094 |
659/1285 (51%) |
540/809 (67%) |
0.58 (0.52‑0.65) |
0.44‑0.65 |
– |
– |
7.5 |
5.9‑9.1; MNE in one trial |
|
Aromatase inhibitor trials |
1111 |
299/639 (47%) |
291/472 (62%) |
0.55 (0.47‑0.65) |
0.44‑0.62 |
21.8 (19.4‑25.3) |
13.0 (11.3‑13.8) |
8.8 |
6.4‑9.1; MNE in one trial |
|
Fulvestrant trials4 |
983 |
360/646 (56%) |
249/337 (74%) |
0.56 (0.47‑0.65) |
0.47‑0.65 |
– |
– |
7.9 |
5.9‑9.1 |
|
Not liver or lung metastases |
|||||||||
|
Overall |
2106 |
533/1331 (40%) |
442/775 (57%) |
0.59 (0.52‑0.67) |
0.50‑0.66 |
– |
– |
11.5 |
5.4‑16.8; MNE in one trial |
|
Aromatase inhibitor trials |
1141 |
253/681 (37%) |
241/460 (52%) |
0.55 (0.46‑0.66) |
0.51‑0.66 |
31.8 (28.1‑39.1) |
19.2 (16.6‑22.3) |
12.6 |
5.4‑16.8; MNE in one trial |
|
Fulvestrant trials4 |
965 |
280/650 (43%) |
201/315 (64%) |
0.59 (0.49‑0.71) |
0.50‑0.64 |
– |
– |
8.1 |
6.3‑10.7 |
|
Age ≤ 40 years |
|||||||||
|
Overall |
282 |
70/164 (43%) |
82/118 (69%) |
0.54 (0.39‑0.75) |
0.35‑0.92 |
– |
– |
8.7 |
3.3‑8.4; MNE in three trials |
|
Aromatase inhibitor trials |
193 |
45/106 (42%) |
61/87 (70%) |
0.50 (0.34‑0.74) |
0.35‑0.92 |
19.8 (16.7‑NE) |
11.2 (7.5‑13.9) |
8.6 |
3.3 in one trial; MNE in three trials |
|
Fulvestrant trials4 |
89 |
25/58 (43%) |
21/31 (68%) |
0.65 (0.54‑0.65) |
0.46‑0.80 |
– |
– |
6.8 |
5.1‑8.4 |
|
Age > 40 years |
|||||||||
|
Overall |
3918 |
1122/2452 (46%) |
900/1466 (61%) |
0.59 (0.54‑0.65) |
0.49‑0.62 |
– |
– |
8.8 |
7.4‑13.3; MNE in one trial |
|
Aromatase inhibitor trials |
2059 |
507/1214 (42%) |
471/845 (56%) |
0.56 (0.49‑0.64) |
0.54‑0.57 |
28.0 (26.2‑30.8) |
15.6 (14.2‑17.0) |
12.4 |
8.0‑13.3; MNE in one trial |
|
Fulvestrant trials4 |
1859 |
615/1238 (50%) |
429/621 (69%) |
0.57 (0.51‑0.65) |
0.49‑0.62 |
– |
– |
7.3 |
7.4‑7.7 |
Abbreviations: CDKI, cyclin-dependent kinase inhibitor; CI, confidence interval; MNE, median not estimable; NE, not estimable; PFS, progression free survival
1 Estimated by a Cox regression model with treatment as the only covariate.
2 Kaplan–Meier estimate, reported only for pooled trials in the first-line setting.
3 Estimated using the Brookmeyer Crowley method by a log-log transform.
4 Results in subgroups are reported only for the pooled Fulvestrant trials, irrespective of the line of therapy, because of insufficient data in the first line setting.
5 The trial showing a −3.9 median difference in PFS with a hazard ratio of 1.07 had a very small sample size of patients with tumors of lobular histology.
Table 3. Progression Free Survival by Race in the Trials of CDKI 4/6 Inhibitors Plus an Aromatase Inhibitor
|
Race |
No. of Patients |
Median PFS, CDKI + AI, Months (95% CI) |
Median PFS, Placebo + AI, Months (95% CI) |
Difference in Estimated Median PFS, Months |
Difference in Estimated Median PFS Range in Individual Trials, Months |
HR (95% CI) |
HR Range in Individual Trials |
|---|---|---|---|---|---|---|---|
|
All patients |
2252 |
28.0 (25.3‑29.1) |
14.9 (14.0‑16.7) |
13.1 |
13.0‑13.3; MNE in one trial |
0.55 (0.49‑0.62) |
0.54‑0.56 |
|
White |
1594 |
26.2 (22.8‑28.1) |
15.6 (14.0‑16.9) |
10.6 |
6.8‑13.2; MNE in one trial |
0.60 (0.52‑0.69) |
0.56‑0.68 |
|
Asian |
438 |
29.1 (26.0‑NE) |
13.0 (9.7‑14.8) |
16.1 |
11.9 in one trial; MNE in three trials |
0.41 (0.31‑0.54) |
0.33‑0.55 |
Abbreviations: CDKI, cyclin-dependent kinase inhibitor; CI, confidence interval; HR, hazard ratio; MNE, median not estimable; NE, not estimable; PFS, progression free survival
Figure 1. Kaplan–Meier Curves of Pooled Analyses of Progression Free Survival with CDKIs or Placebo Plus (A) Aromatase Inhibitor in the First Line Setting or (B) Fulvestrant in the First Line Setting
Abbreviation: CDKI, cyclin-dependent kinase inhibitor