How Modeling Was Used to Support the FDA Approval of a Topical Generic Drug Product
CDER Researchers have been investigating how pharmacokinetic (PK) modeling can support a determination of bioequivalence (BE) for topical drug products. In the case of a generic diclofenac sodium topical gel, 1%, CDER found that physiologically based pharmacokinetic (PBPK) modeling supported an alternative BE approach that does not include a comparative clinical endpoint BE study. The abbreviated new drug application (ANDA) that was approved using this approach is a significant milestone for the Generic Drug User Fee Amendments (GDUFA) regulatory research program that is supported by FDA.
Locally acting drug products deliver their active ingredient at or near the site of action which may be in or on the body. These products include, for example, orally inhaled, nasal, ophthalmic, and topical drug products applied directly to the skin and oral products that act locally in the gastrointestinal tract.1 To demonstrate BE between a brand name product (reference) and its generic (test), there must be an “absence of significant difference in the rate and extent to which the active ingredient or active moiety in pharmaceutical equivalents or pharmaceutical alternatives becomes available at the site of drug action when administered at the same molar dose under similar conditions in an appropriately designed study.”2
FDA’s recent approval of generic diclofenac sodium topical gel, 1%, was a successful example of PBPK modeling supporting an alternative BE approach for an ANDA. This is the first time a virtual BE assessment leveraging dermal PBPK modeling and simulation supported a “totality of evidence” BE approach that led to drug approval. The decision to approve this generic drug was based on 1) qualitative and quantitative sameness and physical and structural similarity to the reference product, 2) an in vivo BE study with PK endpoints, and 3) a virtual BE assessment leveraging dermal PBPK modeling and simulation instead of a comparative clinical endpoint study in patients. Evidence from additional PBPK modeling and simulation activities conducted by CDER assessors bridged the gap between the in vitro evidence for comparable (upstream) bioavailability through the skin and the in vivo evidence for comparable (downstream) bioavailability in the systemic circulation so that a comparative clinical endpoint study was not needed.
The Scientific Challenge
Establishing BE for topical drug products by conducting comparative clinical endpoint studies can be costly, and the studies may not be sufficiently sensitive to detect certain formulation differences. Quantitative methods and modeling (e.g., PBPK) can support alternative BE approaches with reduced or no human testing. However, to be used for regulatory decision-making, these mathematical models need to be sufficiently verified and validated (V&V) for their intended purpose.
Verification and Validation of a Dermal PBPK model for Diclofenac Sodium Topical Gel, 1%
For this ANDA, the applicant implemented an innovative two-level approach for verifying and validating the fit-for-purpose dermal PBPK model developed for diclofenac sodium topical gel, 1%. Briefly, the first level V&V focused on assessing the performance of the developed models for topical products with diclofenac sodium as the active pharmaceutical ingredient (API). The dermal PBPK model for diclofenac sodium topical gel, 1%, incorporated product-specific formulation attributes generated by the applicant as part of its product characterization program and was validated leveraging clinical PK data supporting the ANDA submission.
The second-level V&V aimed at assessing the performance of the computational platform that was used (i.e., the Multi-Phase Multi-Level Mechanistic Dermal Absorption model within the Simcyp® Simulator, version 17). The performance of the platform was evaluated by the ANDA applicant and CDER assessors validating a significant number of PBPK models for dermatological products using published data. The dermal PBPK models supporting the second-level validation included dermatological products with APIs other than diclofenac sodium, as these were covered under the first-level validation. The overview of the model validation process is presented in Figure 1.
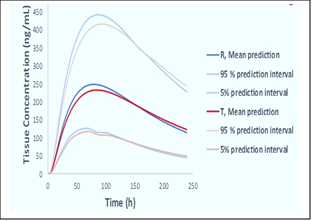
Figure 1. Overview of the V&V methodology proposed by the applicant in support of their fit-for-purpose dermal PBPK models for Voltaren topical gel, 1% (reference, R) and for the generic diclofenac sodium topical gel, 1% (test, T).
Published literature sources 3-7 were used by the applicant and the Agency to validate the dermal PBPK models developed for diclofenac sodium topical drug products, including models for solution and gel (emulsion) formulations. Simulation scenarios captured model performance following a single or multiple product application. Model predictions were generated for a range of applied doses at multiple application sites (e.g., the back and the knee) and were compared with empirical data. The applicant validated their product-specific models developed for the reference and the test products by using an independent dataset of the in vivo BE study with PK endpoints that were generated in support of the ANDA. The model was developed based on a “bottom-up” approach. Although sensitivity analysis-guided parameter optimization may have resulted in improved model predictions when compared to observed data extracted from the in vivo BE study with PK endpoints supporting the ANDA, additional modeling work conducted internally by CDER assessors showed that the BE outcome would not be impacted by modest changes in certain model parameters.
As shown in Figure 2, the population predictions for the reference and test drug products were informed by the differing formulation attributes (such as apparent viscosity, formulation pH and oil droplet distribution among others) and the variability assigned to model parameters (physiology, PK, and drug product parameters) within the Simcyp® Simulator. The two products were found to be bioequivalent.
How does this research improve generic drug development and support their approval?
CDER encourages applicants for generic drug products to consider novel quantitative tools as described in Agency guidances, draft guidances, and product specific guidances when submitting their ANDAs. Recently, a PBPK modeling and simulation approach for a generic diclofenac sodium topical gel, 1%, supported an alternative BE approach in lieu of the comparative clinical endpoint BE study recommended in the product-specific guidance. This led to the first ANDA approval for which a PBPK model supported the BE assessment and approval of a generic topical gel. The applicant utilized the pre-ANDA Program to obtain the Agency’s feedback on the proposed alternative BE approach, which the applicant incorporated in their original submission. In this case, the Agency was able to review and approve the generic diclofenac sodium topical gel, 1%, in the first review cycle of this ANDA.
Outcome
FDA approved a generic diclofenac sodium topical gel, 1%, under ANDA 211253 during its first review cycle. This was the first ANDA approval for which a PBPK model supported the BE assessment. The capability of the model to generate local and systemic diclofenac exposure predictions following the application of topical products that were sensitive to formulation differences was adequately validated. The development and enhancements of the modeling platform (i.e., Multi-Phase Multi-Level Mechanistic Dermal Absorption model within the Simcyp® Simulator) used for the development and V&V of the proposed model and the in vitro characterization-based BE methods were partially supported by GDUFA I and GDUFA II research grants. The applicant utilized the pre-ANDA program to obtain the Agency’s feedback on their alternative to the BE approach as found in the FDA’s Draft Guidance on Diclofenac Sodium, which was incorporated in the final ANDA submission. Final approval of the generic diclofenac sodium topical gel, 1%, provides a successful example of the utilization of novel quantitative modeling tools which were researched and published by FDA towards an alternative BE approach for topical generic drug products leading to an ANDA approval. The benefit of applying PBPK models to support regulatory decision-making, especially for locally acting products, warrants further investment, collaboration and joint effort from the Agency, industry, and academia.
This Impact Story is based on: Tsakalozou E, Babiskin A, Zhao L. Physiologically-based pharmacokinetic modeling to support bioequivalence and approval of generic products: A case for diclofenac sodium topical gel, 1. CPT Pharmacometrics Syst Pharmacol. 2021 May;10(5):399-411. doi: 10.1002/psp4.12600. Epub 2021 Mar 9. PMID: 33547863; PMCID: PMC8129718. This approval would not have been possible without the efforts of staff in the Office of Generic Drugs and Office of Pharmaceutical Quality in the development of in vitro characterization methods for topical products, the assessment of the data submitted in the ANDA and the integrated conclusion about the acceptability of the BE evidence.
References
-
Lionberger RA. FDA critical path initiatives: opportunities for generic drug development. AAPS J. 2008; 10: 103– 109.
-
CFR. - Code of Federal Regulations Title 21, Volume 5, Revised as of April 1, 2019. Available from CFR - Code of Federal Regulations Title 21 (fda.gov) [Accessed 10/27/2021]
-
Radermacher J, Jentsch D, Scholl MA, Lustinetz T, Frolich JC. Diclofenac concentrations in synovial fluid and plasma after cutaneous application in inflammatory and degenerative joint disease. Br J Clin Pharmacol. 1991; 31: 537– 541.
-
Brunner M, Davies D, Martin W, Leuratti C, Lackner E, Muller M. A new topical formulation enhances relative diclofenac bioavailability in healthy male subjects. Br J Clin Pharmacol. 2011; 71: 852– 859.
-
Dehghanyar P, Mayer BX, Namiranian K, Mascher H, Muller M, Brunner M. Topical skin penetration of diclofenac after single- and multiple-dose application. Int J Clin Pharmacol Ther. 2004; 42: 353-359
-
Sioufi A, Pommier F, Boschet F, Godbillon J, Lavoignat D, Salliere D. Percutaneous absorption of diclofenac in healthy volunteers after single and repeated topical application of diclofenac Emulgel. Biopharm Drug Dispos. 1994; 15: 441– 449.
-
Seth BL. Comparative pharmacokinetics and bioavailability study of percutaneous absorption of diclofenac from two topical formulations containing drug as a solution gel or as an emulsion gel. Arzneimittelforschung. 1992; 42: 120– 122.