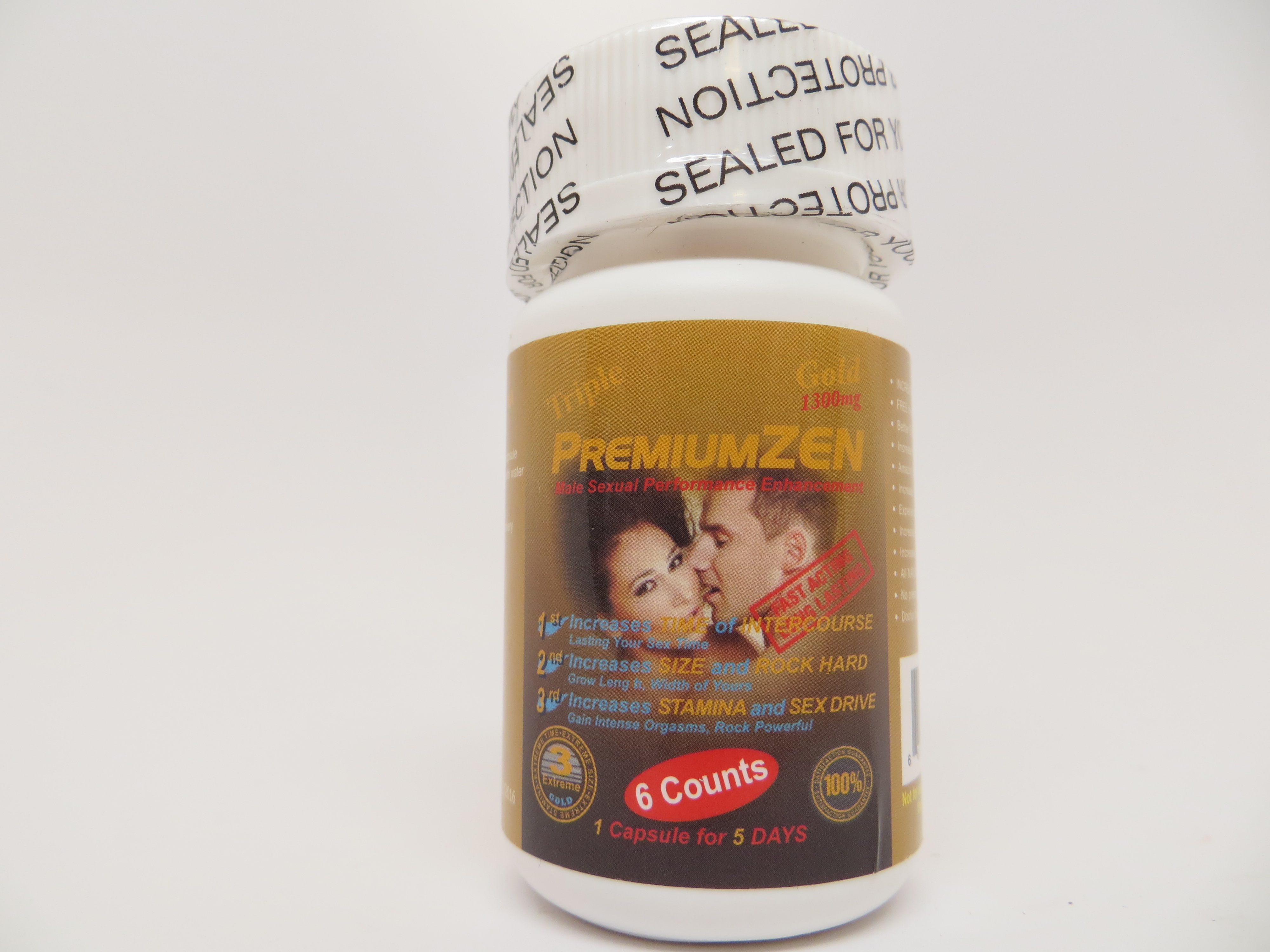
Public Notification: Triple Premium Zen Gold 1300mg Contains Hidden Drug Ingredients
[6/23/2017] The Food and Drug Administration is advising consumers not to purchase or use Triple Premium Zen Gold 1300mg, a product manufactured by SHH Trading and promoted for sexual enhancement on various websites, and possibly sold in some retail stores.
FDA laboratory analysis confirmed that Triple Premium Zen Gold 1300mg contains sildenafil, tadalafil and dapoxetine. Sildenafil and tadalafil, the active ingredients in the FDA-approved prescription drugs Viagra and Cialis, respectively, are used to treat erectile dysfunction. These undeclared ingredients may interact with nitrates found in some prescription drugs such as nitroglycerin and may lower blood pressure to dangerous levels. Men with diabetes, high blood pressure, high cholesterol, or heart disease often take nitrates. Dapoxetine is an active ingredient not approved by FDA, and therefore its safety and efficacy have not been established.
Health care professionals and patients are encouraged to report adverse events or side effects related to the use of this product to FDA's MedWatch Safety Information and Adverse Event Reporting Program:
- Complete and submit the report online at MedWatch Online Voluntary Reporting Form, or;
- Download and complete the form, then submit it via fax at 1-800-FDA-0178.
Note: This notification is to inform the public of products marketed as dietary supplements or conventional foods with hidden drugs and chemicals. These products are typically promoted for sexual enhancement, weight loss, and body building and are often represented as being “all natural.” FDA is unable to test and identify all products marketed as dietary supplements that have potentially harmful hidden ingredients. Consumers should exercise caution before purchasing any product in the above categories.
For more information:
Resources For You
- Sign up to receive email updates about Tainted Products Sold as Dietary Supplements
- Subscribe to Tainted Dietary Supplements RSS Feed