Drug Trials Snapshots: REYVOW
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the REYVOW Package Insert for complete information.
REYVOW (lasmiditan)
Rā – vaü
Elly Lilly and Company
Approval date: October 11, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
REYVOW is a drug used for the acute treatment of migraine with or without aura in adults.
A migraine is a type of headache that, in addition to pain, can be associated with nausea, vomiting, and/or sensitivity to light or sound. Patients who suffer from migraines with aura develop temporary visual or other signs and symptoms shortly before or at the time of headache onset.
How is this drug used?
REYVOW is a tablet taken by mouth, as needed.
What are the benefits of this drug?
A higher percentage of patients who received REYVOW were pain free two hours after the treatment, in comparison to patients who received placebo. Also, a higher percentage of patients who received REYVOW were free of their most bothersome migraine associated symptoms (such as light sensitivity, sound sensitivity, or nausea) two hours after the treatment, in comparison to patients who received placebo.
What are the benefits of this drug (results of trials used to assess efficacy)?
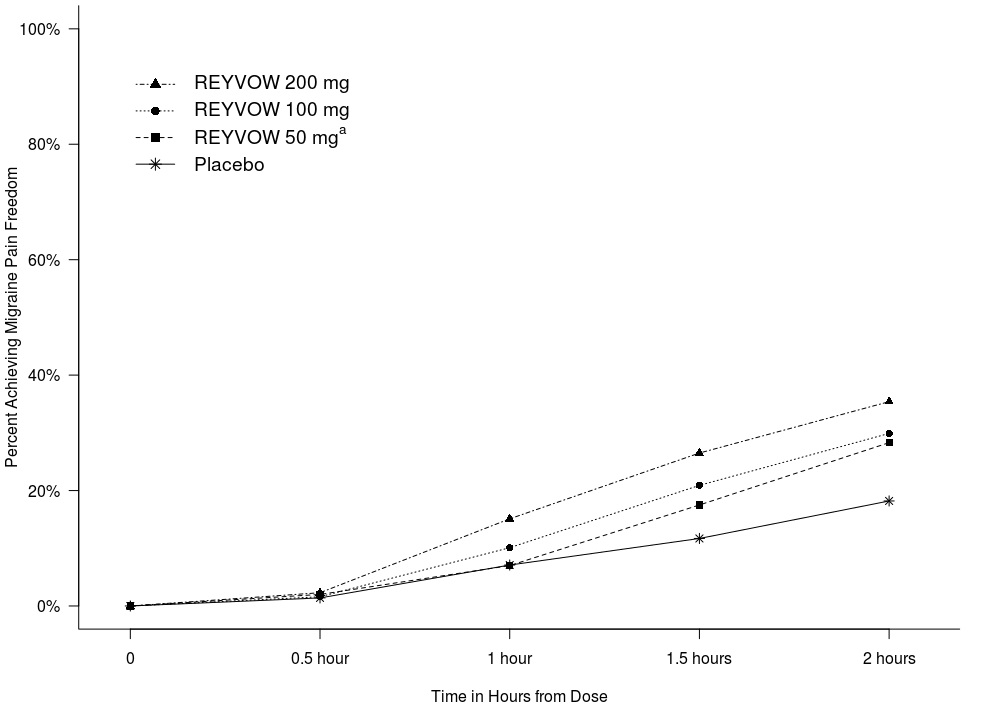
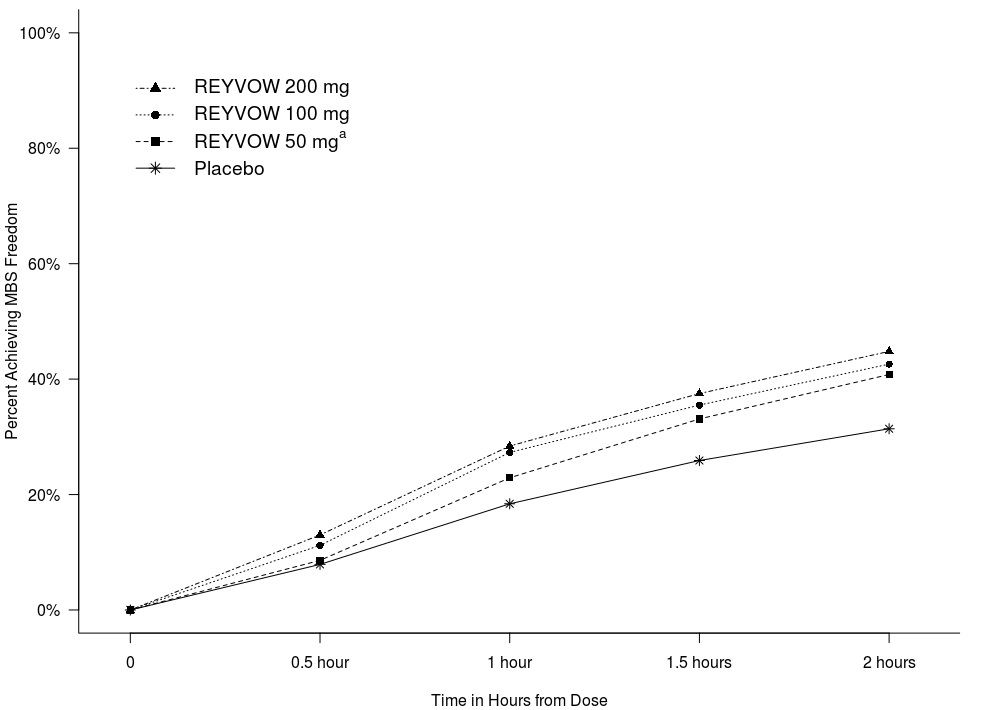
The figures below summarize efficacy results for the evaluated patients in Trials 1 and 2. The primary outcome was the percentage of patients achieving pain freedom and the percentage of patients achieving most bothersome symptom (MBS) freedom 2 hours after treatment.
Figure 4: Percentage of Patients Achieving Migraine Pain Freedom within 2 Hours in Pooled Trials 1 and 2
Figure 5: Percentage of Patients Achieving MBS Freedom within 2 Hours in Pooled Trials 1 and 2
aThe 50 mg arm was only included in Trial 2.
REYVOW Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: REYVOW worked similarly in men and women.
- Race: REYVOW worked similarly among racial groups.
- Age: REYVOW worked similarly in patients younger or older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The tables below summarize efficacy results in each trial by age, sex, and race.
Table 2. Subgroup Efficacy Analyses – Trial 1
| Pain Free 2 hours | Total Patients | Success Count | Success Rate | Total Patients | Success Count | Success Rate | Total Patients | Success Count | Success Rate |
|---|---|---|---|---|---|---|---|---|---|
| Age < Median | Age >= Median | ||||||||
| 100 mg | 241 | 76 | 31.54% | 257 | 65 | 25.29% | |||
| 200 mg | 251 | 92 | 36.65% | 252 | 68 | 26.98% | |||
| Placebo | 249 | 42 | 16.87% | 272 | 37 | 13.91% | |||
| Sex = Women | Sex = Male | ||||||||
| 100 mg | 402 | 111 | 27.61% | 96 | 30 | 31.25% | |||
| 200 mg | 424 | 142 | 33.49% | 79 | 18 | 22.78% | |||
| Placebo | 441 | 68 | 15.42% | 74 | 11 | 14.86% | |||
| Race = White | Race = Black | Race = All Other | |||||||
| 100 mg | 384 | 102 | 26.56% | 87 | 31 | 35.63% | 27 | 8 | 29.63% |
| 200 mg | 382 | 122 | 31.94% | 98 | 32 | 32.65% | 23 | 6 | 26.09% |
| Placebo | 414 | 58 | 14.01% | 86 | 20 | 23.26% | 15 | 1 | 6.67% |
| MBS Free 2 hours | Total Patients | Success Count | Success Rate | Total Patients | Success Count | Success Rate | Total Patients | Success Count | Success Rate |
| Age < Median | Age >= Median | ||||||||
| 100 mg | 229 | 96 | 41.92% | 235 | 95 | 40.43% | |||
| 200 mg | 234 | 103 | 44.02% | 233 | 87 | 37.34% | |||
| Placebo | 231 | 72 | 31.17% | 249 | 70 | 28.11% | |||
| Sex = Women | Sex = Men | ||||||||
| 100 mg | 374 | 148 | 39.57% | 90 | 43 | 47.78% | |||
| 200 mg | 396 | 159 | 40.15% | 71 | 31 | 43.66% | |||
| Placebo | 413 | 123 | 29.78% | 67 | 19 | 28.36% | |||
| Race =White | Race=Black | Race=All Other | |||||||
| 100 mg | 362 | 148 | 40.88% | 77 | 31 | 40.26% | 25 | 12 | 48.00% |
| 200 mg | 358 | 151 | 42.18% | 88 | 31 | 35.23% | 21 | 8 | 38.10% |
| Placebo | 389 | 106 | 27.25% | 79 | 33 | 41.77% | 12 | 3 | 25.00% |
Table 3. Subgroup Efficacy Analyses – Trial 2
| Pain Free 2 hours | Total Patients | Success Count | Success Rate | Total Patients | Success Count | Success Rate | Total Patients | Success Count | Success Rate |
|---|---|---|---|---|---|---|---|---|---|
| Age < Median | Age >= Median | ||||||||
| 50 mg | 256 | 74 | 28.91% | 288 | 80 | 27.78% | |||
| 100 mg | 250 | 79 | 31.60% | 273 | 85 | 31.14% | |||
| 200 mg | 255 | 85 | 33.33% | 266 | 117 | 43.98% | |||
| Placebo | 267 | 48 | 17.98% | 267 | 64 | 23.97% | |||
| Sex = Women | Sex = Male | ||||||||
| 50 mg | 473 | 143 | 30.23% | 83 | 16 | 19.28% | |||
| 100 mg | 455 | 139 | 30.55% | 77 | 28 | 36.36% | |||
| 200 mg | 436 | 173 | 39.68% | 92 | 32 | 34.78% | |||
| Placebo | 459 | 95 | 20.70% | 81 | 20 | 24.69% | |||
| Race = White | Race = Black | Race = All Other | |||||||
| 50 mg | 443 | 117 | 26.41% | 80 | 30 | 37.50% | 21 | 7 | 33.33% |
| 100 mg | 428 | 137 | 32.01% | 77 | 21 | 27.27% | 18 | 6 | 33.33% |
| 200 mg | 432 | 166 | 38.43% | 71 | 29 | 40.85% | 18 | 7 | 38.89% |
| Placebo | 442 | 85 | 19.23% | 74 | 23 | 31.08% | 18 | 4 | 22.22% |
| MBS Free 2 hours | Total Patients | Success Count | Success Rate | Total Patients | Success Count | Success Rate | Total Patients | Success Count | Success Rate |
| Age < Median | Age >= Median | ||||||||
| 50 mg | 240 | 91 | 37.92% | 262 | 114 | 43.51% | |||
| 100 mg | 242 | 107 | 44.21% | 249 | 109 | 43.78% | |||
| 200 mg | 241 | 115 | 47.72% | 237 | 118 | 49.79% | |||
| Placebo | 259 | 78 | 30.12% | 250 | 91 | 36.40% | |||
| Sex = Women | Sex = Men | ||||||||
| 50 mg | 429 | 182 | 42.42% | 73 | 23 | 31.51% | |||
| 100 mg | 424 | 181 | 42.69% | 67 | 35 | 52.24% | |||
| 200 mg | 400 | 197 | 49.25% | 78 | 36 | 46.15% | |||
| Placebo | 434 | 142 | 32.72% | 75 | 27 | 36.00% | |||
| Race =White | Race=Black | Race=All Other | |||||||
| 50 mg | 410 | 167 | 40.73% | 71 | 31 | 43.66% | 21 | 7 | 33.33% |
| 100 mg | 403 | 180 | 44.67% | 71 | 31 | 43.66% | 17 | 5 | 29.41% |
| 200 mg | 400 | 196 | 49.00% | 60 | 30 | 50.00% | 18 | 7 | 38.89% |
| Placebo | 420 | 133 | 31.67% | 71 | 30 | 42.25% | 18 | 6 | 33.33% |
FDA Review
What are the possible side effects?
REYVOW may cause significant drowsiness, therefore driving should be avoided until at least 8 hours after taking REYVOW. Other serious side effects include sedation, the potential for medication overuse headache, and serotonin syndrome. Serotonin syndrome is a rare, serious, and potentially life threating condition which may be more likely to occur if REYVOW is used with antidepressants called SSRIs or SNRIs.
The most common side effects of REYVOW are dizziness, fatigue, tingling in extremities (paresthesia), and sleepiness.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes most common adverse reactions that occurred in combined Trials 1 and 2.
Table 4. Adverse Reactions Occurring in ≥2% and at a Frequency Greater than Placebo in Trials 1 and 2
| Adverse Reaction | REYVOW 50 mg N=654 % |
REYVOW 100 mg N=1265 % |
REYVOW 200 mg N=1258 % |
Placebo N=1262 % |
|---|---|---|---|---|
| Dizziness | 9 | 15 | 17 | 3 |
| Fatiguea | 4 | 5 | 6 | 1 |
| Paresthesiab | 3 | 7 | 9 | 2 |
| Sedationc | 6 | 6 | 7 | 2 |
| Nausea and/or Vomiting | 3 | 4 | 4 | 2 |
| Muscle Weakness | 1 | 1 | 2 | 0 |
a Fatigue includes the adverse reaction related terms asthenia and malaise.
b Paresthesia includes the adverse reaction related terms paresthesia oral, hypoesthesia, and hypoesthesia oral.
c Sedation includes the adverse reaction related term somnolence.
REYVOW Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: The occurrence of side effects was similar in racial groups with exception of dizziness occurring more frequently in White and Asian patients.
- Age: The occurrence of overall side effects was similar in patients younger or older than 65 years of age. Dizziness and a larger increase in systolic blood pressure occurred more frequently in patients who were at least 65 years of age compared to patients who were less than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The tables below summarize the occurrence of the most common adverse reactions by subgroup. REYVOW arm includes all patients who received any of the approved doses (50 mg, 100 mg or 200 mg).
Table 5. Adverse Reactions by Sex
| Preferred Term | Women | Men | ||
|---|---|---|---|---|
| REYVOW N = 2656 % |
Placebo N=1070 % |
REYVOW N=521 % |
Placebo N=192 % |
|
| Dizziness | 14.8 | 3.0 | 14.2 | 2.6 |
| Fatigue | 4.0 | 0.6 | 2.7 | 1.0 |
| Nausea | 3.5 | 1.5 | 2.5 | 2.1 |
| Paresthesia | 6.1 | 1.3 | 3.6 | 2.6 |
| Somnolence | 5.8 | 2.4 | 4.2 | 0.5 |
| Vomiting | 1.1 | 0.7 | 0.4 | 0 |
Table 6. Adverse Reactions by Race
| Preferred Term | White | Black or African American | Asian | Other | Multiple | American Indian or Alaska Native | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| REYVOW N=563 % |
Placebo N=229 % |
REYVOW N=26 % |
Placebo N=5 % |
REYVOW N=46 % |
Placebo N=12 % |
REYVOW N=35 % |
Placebo N=11 % |
REYVOW N=21 % |
Placebo N=6 % |
|||
| REYVOW N=2476 % |
Placebo N=995 % |
|||||||||||
| Dizziness | 15.8 | 2.3 | 9.9 | 4.8 | 15.4 | 0 | 15.2 | 16.7 | 14.3 | 9.1 | 9.5 | 0 |
| Fatigue | 4.1 | 0.8 | 2.7 | 0 | 3.8 | 0 | 2.2 | 0 | 0 | 0 | 4.8 | 0 |
| Nausea | 3.5 | 1.4 | 2.8 | 1.7 | 3.8 | 0 | 2.2 | 8.3 | 0 | 9.1 | 9.5 | 0 |
| Paresthesia | 6.8 | 1.6 | 1.1 | 1.3 | 0 | 0 | 8.7 | 0 | 2.9 | 0 | 4.8 | 0 |
| Somnolence | 5.1 | 2.3 | 6.0 | 1.7 | 15.4 | 0 | 10.9 | 0 | 11.4 | 0 | 9.5 | 0 |
| Vomiting | 1.0 | 0.8 | 1.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4.8 | 0 |
Table 7. Adverse Reactions by Age
| Preferred Term | Age < 30 years | ≥ 30 to <50 years | ≥ 50 to <65 years | ≥ 65 years | ||||
|---|---|---|---|---|---|---|---|---|
| REYVOW | Placebo | REYVOW | Placebo | REYVOW | Placebo | REYVOW | Placebo | |
| N = 560 | N=234 | N=1666 | N=639 | N=819 | N=335 | N=132 | N=54 | |
| % | % | % | % | % | % | % | % | |
| Dizziness | 17.3 | 3.8 | 13.9 | 3.0 | 13.8 | 2.4 | 18.9 | 1.9 |
| Fatigue | 2.9 | 0.4 | 3.8 | 0.6 | 4.6 | 0.6 | 2.3 | 1.9 |
| Nausea | 3.6 | 1.3 | 3.2 | 2.0 | 3.7 | 0.6 | 3.0 | 3.7 |
| Paresthesia | 3.8 | 0.9 | 5.2 | 1.4 | 7.6 | 1.8 | 7.6 | 3.7 |
| Somnolence | 5.7 | 0.9 | 5.5 | 2.5 | 5.9 | 2.7 | 2.3 | 0 |
| Vomiting | 0.7 | 0.9 | 1.3 | 0.6 | 0.6 | 0.6 | 0.8 | 0 |
FDA Review
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved REYVOW primarily based on data from 2 clinical trials, Trial 1 (# NCT02439320) and Trial 2 (#NCT02605174) of 4439 patients with migraine headaches with or without aura. Trials were conducted at 224 sites in the United States, the United Kingdom, and Germany.
Demographics of patients who provided data for evaluation of efficacy are presented in Tables 9 and 10 under MORE INFO.
Demographics of patients who provided data for evaluation of side effects (safety population) are presented below.
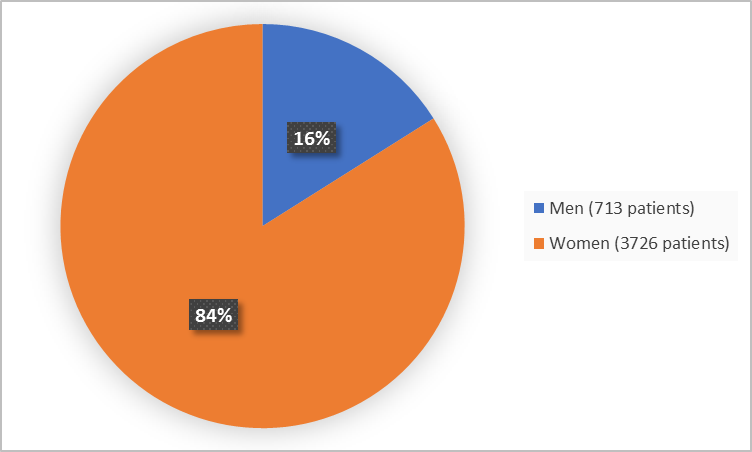
Figure 1 summarizes how many men and women were in the clinical trials used to evaluate safety.
Figure 1. Baseline Demographics by Sex (safety population)
FDA Review
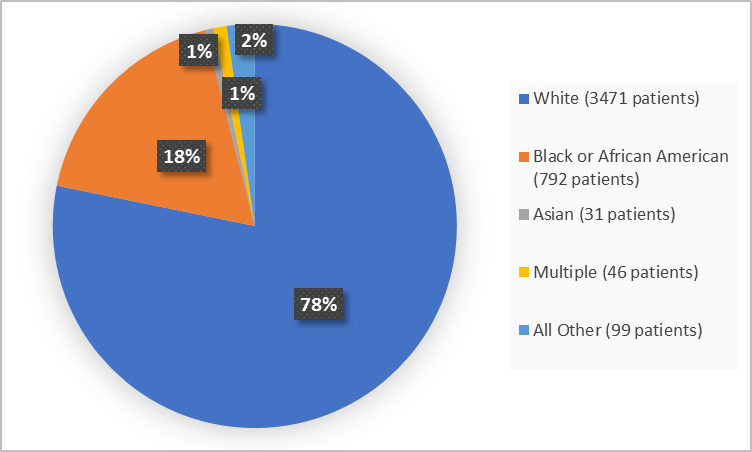
Figure 2 summarizes the percentage of patients by race in the clinical trials used to evaluate safety.
Figure 2. Baseline Demographics by Race (safety population)
Table 1. Demographics of Safety Trials by Race
| Race | Number of Patients | Percentage of Patients |
|---|---|---|
| White | 3471 | 78 |
| Black or African American | 792 | 18 |
| Asian | 31 | 1 |
| American Indian or Alaska Native | 27 | 1 |
| Native Hawaiian or other Pacific Islander | 13 | Less than 1 |
| Multiple | 46 | 1 |
| Other | 59 | 1 |
FDA Review
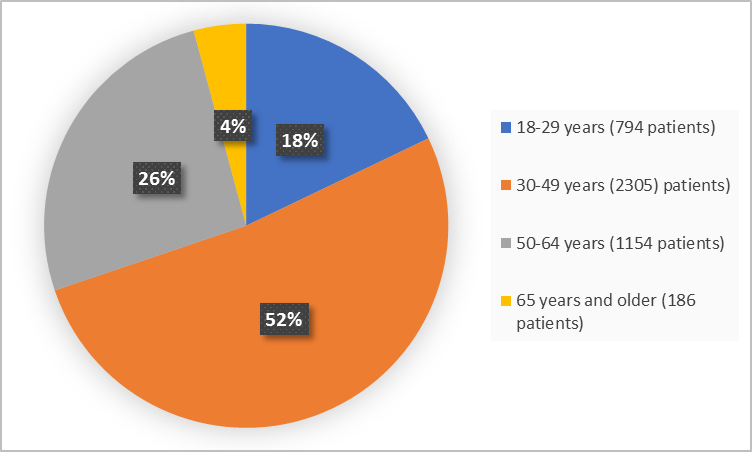
Figure 3. Baseline Demographics by Age (safety population)
FDA Review
Who participated in the trials?
Demographic data for the safety population is presented in Table 8, and data for efficacy populations of individual Trials 1 and 2 in Tables 9 and 10, respectively.
Table 8. Demographic Characteristics (safety population)
| Demographic Parameters | Placebo | REYVOW | TOTALa | ||
|---|---|---|---|---|---|
| N=1262 n (%) | 50 mg N=654 n (%) | 100 mg N=1265 n (%) | 200 mg N=1258 n (%) | N=4439 n (%) | |
| Sex | |||||
| Men | 192 (15.2) | 100 (15.3) | 214 (16.9) | 207 (16.5) | 713 (16.1) |
| Women | 1070 (84.8) | 554 (84.7) | 1051 (83.1) | 1051 (83.6) | 3726 (83.9) |
| Race | |||||
| White | 995 (78.8) | 524 (80.1) | 980 (77.5) | 972 (77.3) | 3471 (78.2) |
| Black or African American | 229 (18.1) | 106 (16.2) | 226 (17.9) | 231 (18.4) | 792 (17.8) |
| Asian | 5 (0.4) | 5 (0.8) | 10 (0.8) | 11 (0.9) | 31 (0.7) |
| American Indian or Alaska Native | 6 (0.5) | 3 (0.5) | 9 (0.7) | 9 (0.7) | 27 (0.6) |
| Native Hawaiian or other Pacific Islander | 4 (0.3) | 2 (0.3) | 2 (0.2) | 5 (0.4) | 13 (0.3) |
| Multiple | 11 (0.9) | 8 (1.2) | 16 (1.3) | 11 (0.9)/td> | 46 (1.0) |
| Other | 12 (1.0) | 6 (0.9) | 22 (1.7) | 18 (1.4) | 58 (1.3) |
| Age | |||||
| Mean years (SD) | 42.5 (12.6) | 42.8 (13.2) | 42.8 (12.2) | 41.6 (12.2) | 42.4 (12.5) |
| Median (years) | 43 | 42 | 43 | 41 | 42 |
| Min, max (years) | 18, 79 | 18, 77 | 18, 78 | 18, 81 | 18, 81 |
| Age Group (years) | |||||
| 18-29 | 234 (18.5) | 119 (18.2) | 206 (16.3) | 235 (18.7) | 794 (17.9) |
| 30-49 | 639 (50.6) | 315 (48.2) | 669 (52.9) | 682 (54.2) | 2305 (51.9) |
| 50-64 | 335 (26.6) | 182 (27.8) | 343 (27.1) | 294 (23.4) | 1154 (26.0) |
| ≥65 | 54 (4.3) | 38 (5.8) | 47 (3.7) | 47 (3.7) | 186 (4.2) |
| Ethnicity | |||||
| Hispanic or Latino | 208 (16.5) | 135 (20.6) | 219 (17.3) | 213 (16.9) | 775 (17.5) |
| Not Hispanic or Latino |
1047 (83.0) | 515 (78.7) | 1037 (82.0) | 1038 (82.5) | 3637 (81.9) |
| Region | |||||
| United States | 1156 (91.6) | 542 (82.9) | 1161 (91.8) | 1151 (91.5) | 4010 (90.3) |
| Germany and UK | 106 (8.4) | 112 (17.1) | 104 (8.2) | 107 (8.5) | 429 (9.7) |
FDA Review
Table 9. Demographic Characteristics (Trial 1- efficacy population)
| REYVOW 100 mg N=498 |
REYVOW 200 mg N=503 |
Placebo N=515 |
TOTAL N=1516 |
|
|---|---|---|---|---|
| Sex, n (%) | ||||
| Women | 402 (80.72%) | 424 (84.29%) | 441 (85.63%) | 1267 (83.6%) |
| Men | 96 (19.28%) | 79 (15.71%) | 74 (14.37%) | 249 (16.4%) |
| Race, n (%) | ||||
| American Indian or Alaska Native | 4 (0.80%) | 5 (0.99%) | 1 (0.19%) | 10 (0.66%) |
| Asian | 2 (0.40%) | 2 (0.40%) | 2 (0.39%) | 6 (0.40%) |
| Black or African American | 87 (17.47%) | 98 (19.48%) | 86 (16.70%) | 271 (17.88%) |
| Native Hawaiian or other Pacific Islander | 1 (0.20%) | 2 (0.40%) | 1 (0.19%) | 4 (0.26%) |
| White | 384 (77.11%) | 382 (75.94%) | 414 (80.39%) | 1180 (77.84%) |
| Other | 10 (2.01%) | 9 (1.79%) | 6 (1.17%) | 25 (1.65%) |
| Multiple | 10 (2.01%) | 5 (0.99%) | 5 (0.97%) | 20 (1.32%) |
| Age (years), n | ||||
| Mean (SD) | 42.06 (11.59) | 41.28 (12.08) | 42.01 (12.44) | 41.79 (12.04) |
| Median | 42.0 | 41.0 | 42.0 | 41.00 |
| Minimum | 18 | 18 | 18 | 18 |
| Maximum | 74 | 72 | 78 | 78 |
Table 10. Demographic Characteristics (Trial 2- efficacy population)
| REYVOW 50 mg N=544 |
REYVOW 100 mg N=523 |
REYVOW 200 mg N=521 |
Placebo N=534 | TOTAL N=2122 | |
|---|---|---|---|---|---|
| Sex, n (%) | |||||
| Women | 462 (84.93%) | 448 (85.66%) | 431 (82.73%) | 454(85.02%) | 1795 (84.59%) |
| Men | 82(15.07%) | 75 (14.34%) | 90 (17.27%) | 80(14.98%) | 327(15.41%) |
| Race, n (%) | |||||
| American Indian or Alaska Native | 3(0.55%) | 2(0.38%) | 0 | 5 (0.94%) | 10 (0.47%) |
| Asian | 5(0.92%) | 5 (0.96%) | 7 (1.35%) | 3 (0.56%) | 20 (0.94%) |
| Black or African American | 80 (14.71%) | 77 (14.72%) | 71 (13.65%) | 74 (13.86%) | 302 (14.24%) |
| Native Hawaiian or other Pacific Islander | 2(0.37%) | 0 | 2 (0.38%) | 2 (0.37%) | 6 (0.28%) |
| White | 443 (81.43%) | 428 (81.84%) | 432 (83.08%) | 442 (82.77%) | 1745 (82.27%) |
| Other | 4(0.74%) | 6 (1.15%) | 4 (0.77%) | 3 (0.56%) | 17 (0.80%) |
| Multiple | 7(1.29%) | 5 (0.96%) | 4 (0.77%) | 5 (0.94%) | 21 (0.99%) |
| Age (years), n | |||||
| Mean (SD) | 42.82(13.28) | 42.54 (12.33) | 41.75 (12.22) | 42.80 (12.74) | 42.72 (12.66) |
| Median | 42 | 44.0 | 42.0 | 42.5 | 43.00 |
| Minimum | 18 | 18 | 18 | 18 | 18 |
| Maximum | 77 | 77 | 79 | 77 | 79 |
FDA Review
How were the trials designed?
The FDA approved REVYOW based primarily on data from two clinical trials of adult patients with a history of migraine with or without aura.
The design of Trials 1 and 2 was similar. Adult patients with migraine were assigned to receive one of two (Trial 1) or three (Trial 2) doses of REYVOW or placebo within 4 hours of the onset of a moderate to severe migraine attack. Patients had to report on the status of their pain and their associated migraine symptoms (including light or sound sensitivity or nausea). Neither the patients nor the health care providers knew which treatment was being given until after the trial was completed.
The benefit of REYVOW was assessed based on the percentage of patients who became pain free within 2 hours and comparing it with placebo treated patients. The assessment also included the percentage of patients who were free of their associated most bothersome migraine symptom within 2 hours of taking the trial drug.
Patients who completed Trials 1 and 2 could enroll in one additional trial. They were randomized to either 100 mg or 200 mg of REYVOW and could treat all migraine attacks for up to 12 months. The data from this trial were used to assess the long-term side effects of REYVOW.
How were the trials designed?
The efficacy of REYVOW in the acute treatment of migraine was evaluated in two randomized, double-blind, placebo-controlled trials which enrolled adult patients with a history of migraine with and without aura.
The primary efficacy analyses were conducted in patients who treated a migraine of moderate to severe intensity within 4 hours of the onset of the attack. The efficacy of REYVOW was assessed on pain freedom at 2 hours and MBS freedom at 2 hours after dosing compared to placebo. Pain freedom was defined as a reduction of moderate or severe headache pain to no pain, and MBS freedom was defined as the absence of the self-identified MBS (photophobia, phonophobia, or nausea).
Patients who completed the first two trials were eligible to enter the long-term open-label safety trial. Patients were able to take REYVOW intermittently as needed for migraine attacks for up to 12 months w (either 100 mg or 200 mg).
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.