Drug Trials Snapshots: XIIDRA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to XIIDRA Prescribing Information for complete information.
XIIDRA (lifitegrast)
(ZYE-druh)
Shire US Inc.
Approval date: July 11, 2016
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
XIIDRA is a drug for the treatment of the signs and symptoms of dry eye disease.
How is this drug used?
One drop of XIIDRA is applied in each eye 2 times each day (1 drop in the morning and 1 drop in the evening).
What are the benefits of this drug?
XIIDRA improved the signs and symptoms of dry eye.
What are the benefits of this drug (results of trials used to assess efficacy)?
The figure below summarizes efficacy results for corneal irritation using Inferior Fluorescein Corneal Staining Score (ICSS),where a score of 0 represents no staining, and a score of 4 represents severe staining in the superior, central, and inferior corneal zones). Results for each trial are presented separately.
Figure 4: Mean Change (SD) from Baseline and Treatment Difference (Xiidra – Vehicle) in Inferior Corneal Staining Score in 12-Week Studies in Patients with Dry Eye Disease.
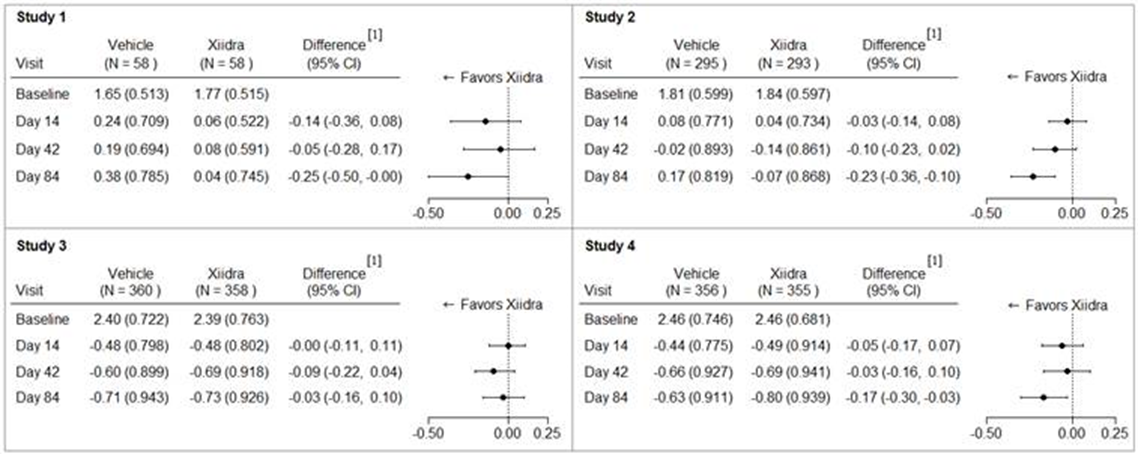
[1] Based on ANCOVA model adjusted for baseline value in Study 1, and ANCOVA model adjusted for baseline value and randomization stratification factors in Studies 2-4. All randomized and treated patients were included in the analysis and missing data were imputed using last-available data. In Study 2, one Vehicle treated subject who did not have a study eye designated was excluded from analysis.
XIIDRA Prescribing Information
The figure below summarizes efficacy results for eye dryness in each trial separately. Eye dryness Score (EDS) was rated by patients using a visual analogue scale (VAS) (0 = no discomfort, 100 = maximal discomfort) at each trial visit.
Figure 5: Mean Change (SD) from Baseline and Treatment Difference (Xiidra – Vehicle) in Eye Dryness Score in 12-Week Studies in Patients with Dry Eye Disease
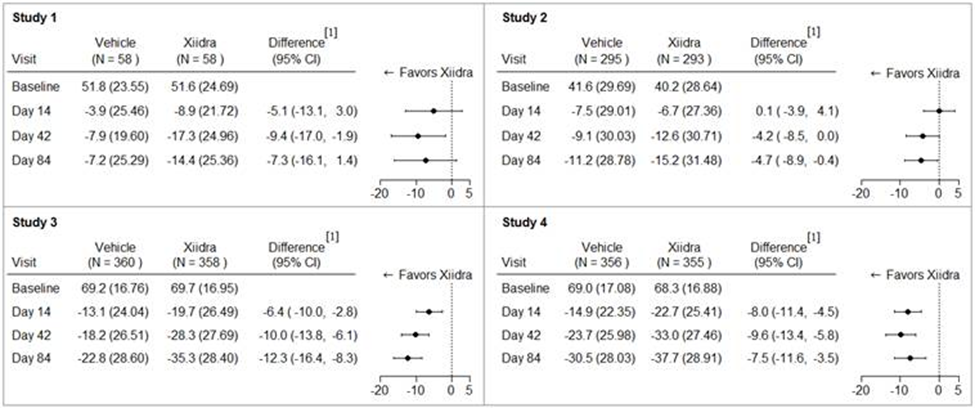
[1] Based on ANCOVA model adjusted for baseline value in Study 1, and ANCOVA model adjusted for baseline value and randomization stratification factors in Studies 2-4. All randomized and treated patients were included in the analysis and missing data were imputed using last-available data. In Study 1, one Xiidra treated subject who did not have a baseline value was excluded from analysis.
XIIDRA Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: XIIDRA worked similarly in men and women.
- Race: XIIDRA worked similarly in White and African American patients. The number of patients in other races was limited, therefore differences in response could not be determined.
- Age: XIIDRA worked similarly in patients above and below age 65.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Subgroup differences for the mean change in Inferior Corneal Staining Score (ICSS) and Eye Dryness Score (EDS) for each trial are presented in the figures below. In some subgroups, there were only a small number of patients in each trial and this may not be the same as the overall effect.
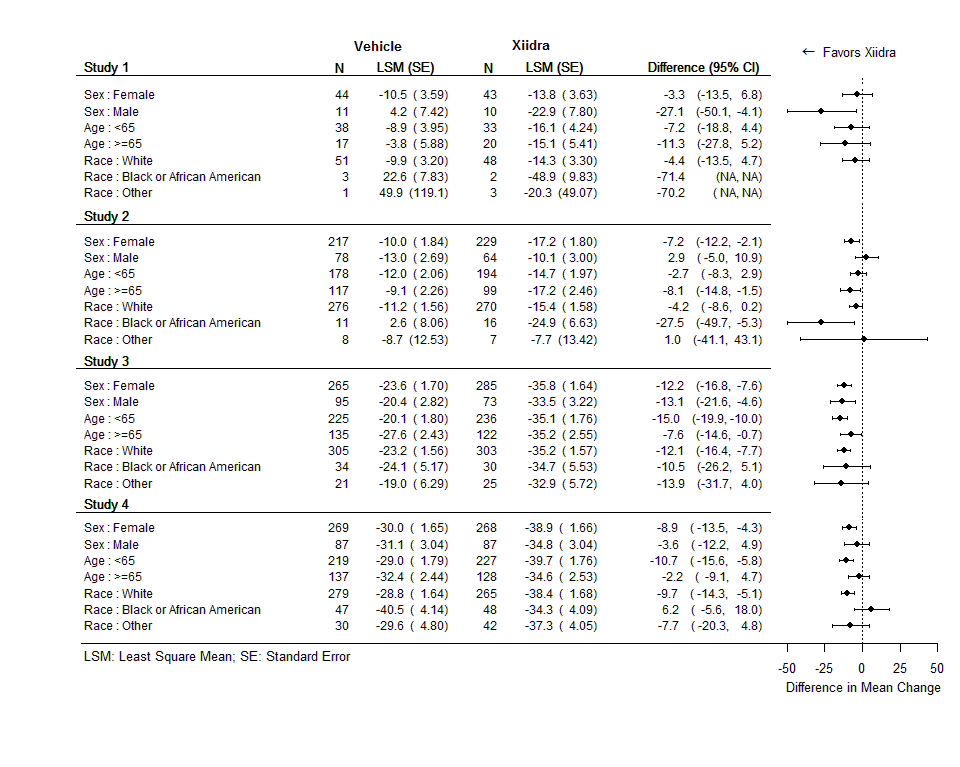
Figure 6. Mean Change (±SE) in ICSS from Baseline at Day 84 by Subgroup (ITT Population, LOCF)
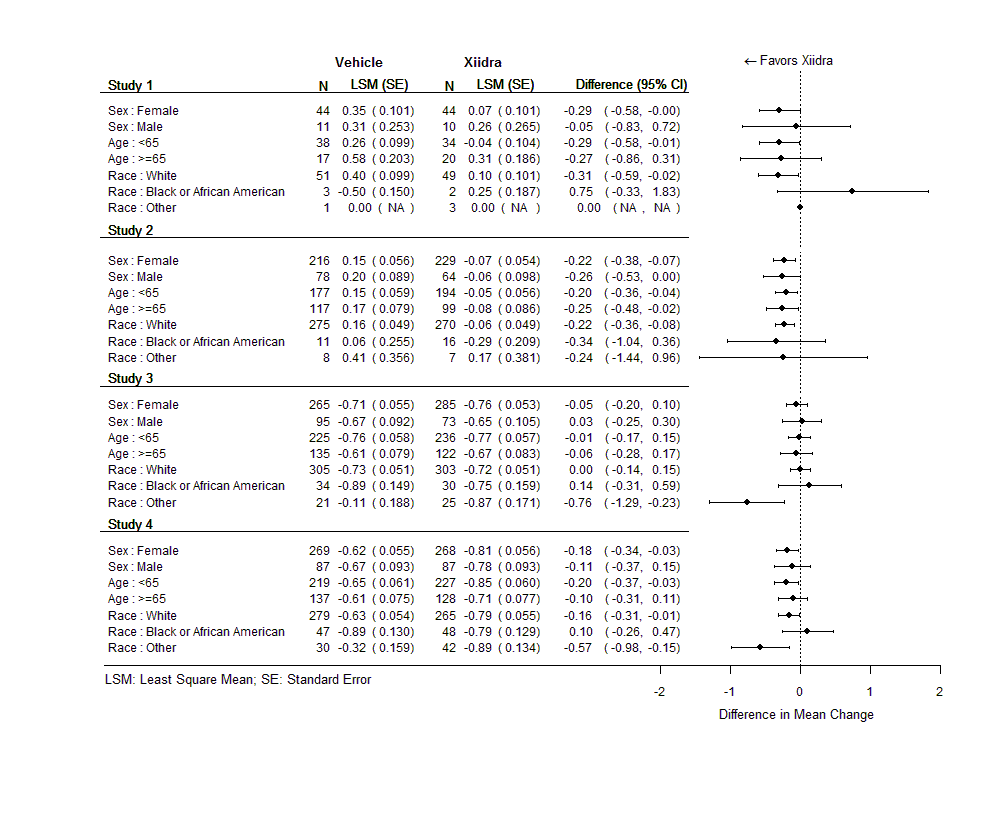
FDA Statistical review
Figure 7. Mean Change (±SE) in EDS from Baseline at Day 84 by Subgroup (ITT Population, LOCF)
FDA Statistical review
What are the possible side effects?
The most common side effects are eye irritation, unusual taste sensation (dysgeusia), and blurred vision.
What are the possible side effects (results of trials used to assess safety)?
The most common adverse reactions reported in 5-25 % of patients were instillation site irritation, dysgeusia and reduced visual acuity.
Other adverse reactions reported in 1% to 5% of the patients were blurred vision, conjunctival hyperemia, eye irritation, headache, increased lacrimation, eye discharge, eye discomfort, eye pruritus and sinusitis.
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: The majority of patients in the trials were white. Differences in side effects among races could not be determined.
- Age: The occurrence of side effects was similar in patients above and below age 65.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes ocular adverse events in treated subgroups for combined four trials
Table 2. Ocular Adverse Events by Subgroups (randomized population)
| XIIDRA N=1067 n/N (%) | Placebo N=1069 n/N (%) | ||
|---|---|---|---|
| Overall | 476/1067 (45) | 212/1069 (20) | |
| Sex | |||
| Men | 96/236 (41) | 53/272 (20) | |
| Women | 380/831 (46) | 159/794 (20) | |
| Age (years) | |||
| 65> | 290/693 (42) | 128/661 (19) | |
| ≥65 | 186/374 (50) | 84/405 (21) | |
| Race | |||
| White | 411/894 (46) | 186/911 (20) | |
| All Other* | 65/173 (38) | 26/155 (17) | |
*Duo to small number of patients in subgroups, all non-White patients are grouped together.
Clinical trial data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved XIIDRA based on evidence from four clinical trials that enrolled 2133 patients with dry eyes. The trials were conducted in the United States.
Figure below summarizes how many men and women were enrolled in the clinical trials.
Figure 1. Baseline Demographics by Sex {randomized population}
Clinical trial data
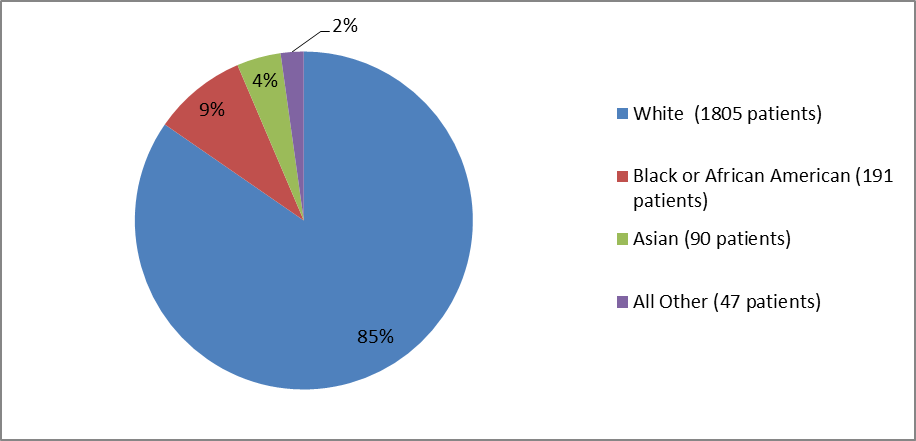
Figure 2 and Table 1 summarize the percentage of patients by race enrolled in the clinical trials.
Figure 2. Baseline Demographics by Race {randomized population}
Clinical trial data
Table 1. Demographics by Race
| Race | Number of Participants | Percentage |
|---|---|---|
| White | 1805 | 85 |
| Black or African American | 191 | 9 |
| Asian | 90 | 4 |
| Native Hawaiian or Pacific Islander | 8 | less than 1% |
| American Indian or Alaskan Native | 11 | 1 |
| Other | 28 | 1 |
Clinical trial data
Figure 3. Baseline Demographics by Age {randomized population}
Clinical trial data
Who participated in the trials?
Table 3. Baseline Demographics (randomized population)
| Trial 1 | Trial 2 | Trial 3 | Trial 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Placebo (N = 58) | XIIDRA (N=58) | Placebo (N = 295) | XIIDRA (N =293) | Placebo (N = 358) | XIIDRA (N = 360) | Placebo (N = 356) | XIIDRA (N = 355) | |
| Age | ||||||||
| Mean (SD) | 60.4 (12.9) | 62.3 (12.2) | 61.1 (11.8) | 60.2 (12.2) | 58.9 (14.3) | 58.7 (13.9) | 58.6 (14.8) | 58.8 (14.1) |
| Median | 60.5 | 62.0 | 62.0 | 59.0 | 60.5 | 59.4 | 61.0 | 60.0 |
| Range | 26.0, 89.0 | 31.0, 85.0 | 24.0, 85.0 | 20.0, 91.0 | 19.8, 88.7 | 19.2, 97.5 | 18.0, 93.0 | 19.0, 87.0 |
| Age Group, n (%) | ||||||||
| 40 (69.0) | 35 (60.3) | 178 (60.3) | 194 (66.2) | 223 (62.5) | 238 (65.9) | 219 (61.5) | 227 (63.9) | |
| ≥65 | 18 (31.0) | 23 (39.7) | 117 (39.7) | 99 (33.8) | 135 (37.5) | 122 (34.1) | 137 (38.5) | 128 (36.1) |
| Sex, n (%) | ||||||||
| Female | 45 (77.6) | 47 (81.0) | 217 (73.6) | 229 (78.2) | 265 (73.6) | 285 (79.6) | 269 (75.6) | 268 (75.5) |
| Male | 13 (22.4) | 11 (19.0) | 78 (26.4) | 64 (21.8) | 95 (26.4) | 73 (20.4) | 87 (24.4) | 87 (24.5) |
| Ethnicity, n (%) | ||||||||
| Hispanic or Latino | 0 (0.0) | 0 (0.0) | 7 (2.4) | 6 (2.0) | 64 (17.8) | 79 (22.1) | 58 (16.3) | 60 (16.9) |
| Not Hispanic or Latino | 58 (100) | 58 (100) | 288 (97.6) | 287 (98.0) | 296 (82.2) | 279 (77.9) | 298 (83.7) | 295 (83.1) |
| Race, n (%) | ||||||||
| American Indian or Alaskan Native | 0 (0.0) | 0 (0.0) | 2 (0.7) | 1 (0.3) | 2 (0.6) | 4 (1.1) | 0 (0.0) | 2 (0.6) |
| Asian | 1 (1.7) | 3 (5.2) | 2 (0.7) | 3 (1.0) | 14 (3.9) | 19 (5.3) | 24 (6.7) | 24 (6.8) |
| Black or African American | 3 (5.2) | 2 (3.4) | 11 (3.7) | 16 (5.5) | 34 (9.4) | 30 (8.4) | 47 (13.2) | 48 (13.5) |
| Native Hawaiian or Other Pacific islander | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (0.8) | 2 ( 0.6) | 1 (0.3) | 2 (0.6) |
| White | 54 (93.1) | 53 (91.4) | 276 (93.6) | 270 (92.2) | 305 (84.7) | 303 (84.6) | 279 (78.4) | 265 (74.6) |
| Other | 0 (0.0) | 0 (0.0) | 4 (1.4) | 3 (1.0) | 2 (0.6) | 0 (0.0) | 5 (1.4) | 14 (3.9) |
FDA Statistical review
How were the trials designed?
The benefits and side effects of XIIDRA were evaluated in four clinical trials. All four trials were similar in design with patients receiving either an XIIDRA or a placebo solution two times a day for 12 weeks. Neither the patients nor the health care providers knew which treatment was being given until after the trials were completed.
The trials measured the improvement in signs of dry eye disease (using Inferior Fluorescein Corneal Staining Score) and in patient’s symptoms (using Eye Dryness Score).
How were the trials designed?
The safety and efficacy of XIIDRA for the treatment of dry eye disease were assessed in in four 12 week, randomized, multi center, double blind, vehicle controlled trials. Patients were randomized to XIIDRA or placebo in a 1:1 ratio.
Signs of dry eye disease were assessed using Inferior Fluorescein Corneal Staining Score (ICSS) (0 = no staining, 1 = few/rare punctate lesions, 2 = discrete and countable lesions, 3 = lesions too numerous to count but not coalescent, 4 = coalescent) at each trial visit.
Symptoms were assessed using Eye Dryness Score (EDS) rated by patients using a visual analogue scale (VAS) (0 = no discomfort, 100 = maximal discomfort) and recorded at each trial visit.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.