Drug Trials Snapshots: VILTEPSO
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to VILTEPSO Prescribing Information for complete information.
VILTEPSO (viltolarsen)
vil tep’ soe
NS Pharma
Approval date: August 12, 2020
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
VILTEPSO is a drug for the treatment of a particular type of Duchenne muscular dystrophy (DMD). It is to be used only in patients who have a specific mutation of the dystrophin gene.
DMD is a rare disease that primarily affects boys. It is caused by low levels of a muscle protein called dystrophin. The lack of dystrophin causes progressive muscle weakness and premature death.
How is this drug used?
VILTEPSO is given by a health care professional once every week directly into the bloodstream through a needle in the vein. This is known as an intravenous, or IV infusion. It takes about one hour to receive VILTEPSO infusion.
What are the benefits of this drug?
VILTEPSO increased levels of dystrophin in the muscles of treated patients. It is believed that this increase may predict clinical benefit in patients.
VILTEPSO was approved under FDA’s accelerated approval program, which provides earlier patient access to a promising new drug while the company continues to conduct clinical trials to confirm that the drug works well.
More trials are ongoing to assess whether there is a clinical benefit of VILTEPSO.
What are the benefits of this drug (results of trials used to assess efficacy)?
The tables below present dystrophin levels at baseline prior to treatment and at Week 25 from patients in Trial 1.
Table 1. Dystrophin Expression in Individual Patients (Trial 1)
| Patient Number | Western Blot % Normal Dystrophina | ||
|---|---|---|---|
| Baseline | Week 25 | Change from Baseline | |
| 1 | 0.46 | 1.14 | 0.69 |
| 2 | 0.40 | 3.97 | 3.57 |
| 3 | 0.46 | 2.97 | 2.51 |
| 4 | 0.09 | 10.40 | 10.31 |
| 5 | 0.51 | 14.42 | 13.91 |
| 6 | 2.61 | 7.40 | 4.79 |
| 7 | 0.43 | 3.06 | 2.63 |
| 8 | 0.09 | 4.07 | 3.98 |
a Data were normalized by myosin heavy chain.
VILTEPSO Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
The trial that looked at the benefit of VILTEPSO was small and consisted of boys only who were of similar age and predominantly White. It was not possible to determine if there were any differences in how well the drug worked in sex, race and age subgroups.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The population for this trial was both small and relatively homogeneous; therefore, no subpopulation efficacy analyses were conducted.
What are the possible side effects?
Kidney damage was observed in animal studies with VILTEPSO.
The most common side effects of VILTEPSO are upper respiratory infections, injection site reactions, cough, and fever.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions that occurred in patients treated with VILTEPSO at recommended dose from pooled Trials 1 and 2.
Table 2. Adverse Reactions Reported in ≥10% of DMD Patients Treated with VILTEPSO 80 mg/kg Once Weekly (Pooled Trials 1 and 2)
| Adverse Reaction | VILTEPSO 80 mg/kg Once Weekly (n=16) % |
|---|---|
| Upper respiratory tract infection | 63 |
| Injection site reaction* | 25 |
| Cough | 19 |
| Pyrexia | 19 |
| Contusion | 13 |
| Arthralgia | 13 |
| Diarrhea | 13 |
| Vomiting | 13 |
| Abdominal pain | 13 |
| Ejection fraction decreased | 13 |
| Urticaria | 13 |
*Injection site reaction includes the following terms: catheter site swelling, infusion site discomfort, infusion site pain, injection site bruising, injection site erythema, injection site extravasation, injection site pain, injection site reaction, injection site swelling.
VILTEPSO Prescribing Information
Were there any differences in side effects among sex, race and age?
The trials that looked at the side effects of VILTEPSO were small and consisted of boys only, who were of similar age and either White or Asian. It was not possible to determine if there were any differences in side effects in sex, race and age subgroups.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The safety population consisted of small and relatively homogeneous population; therefore, no subpopulation safety analyses were conducted.
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the trials?
There were two clinical trials that provided data for VILTEPSO approval. Trials enrolled patients with DMD (Trial 1/NCT02740972 and Trial 2). Trial 1 was conducted at 6 sites in the United States and Canada and Trial 2 at 5 sites in Japan.
Only Trial 1 provided data for evaluation of VILTEPSO benefits. Demographics of that population (called efficacy population) are presented in Table 3 under MORE INFO.
Demographics of the combined populations from both trials that provided data for evaluation of side effects (called safety population) are presented below and in Table 3 under MORE INFO.
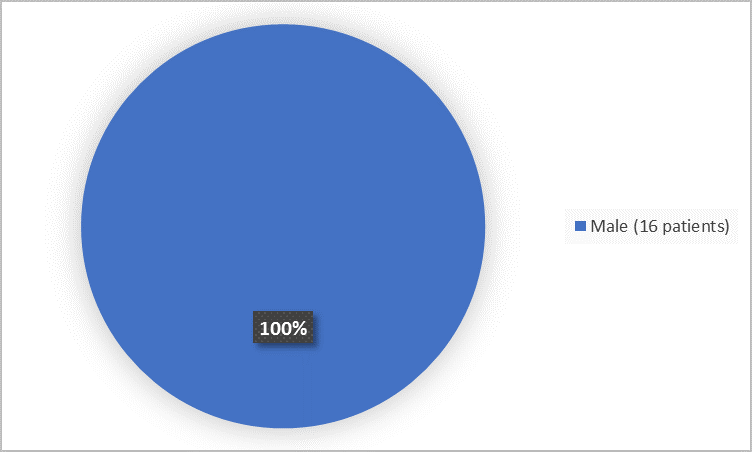
The figure below summarizes patients by sex.
Figure 1. Baseline Demographics by Sex (safety population)
FDA Review
Figure 2 summarizes the percentage of patients by race.
Figure 2. Baseline Demographics by Race (safety population)
FDA Review
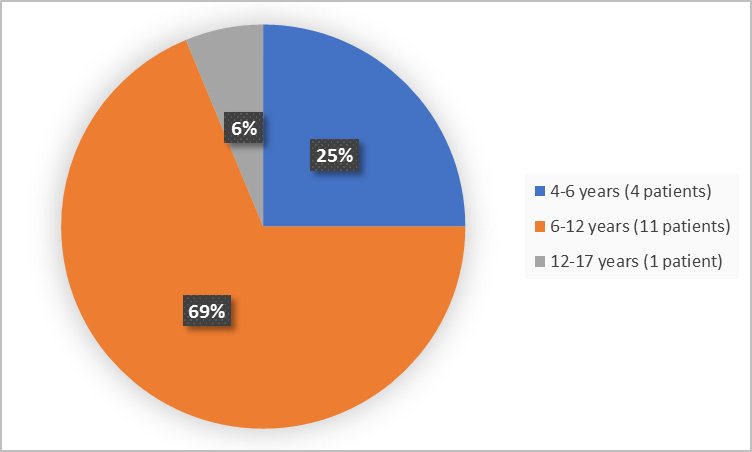
Figure 3 summarizes the percentage of patients by age.
Figure 3. Baseline Demographics by Age (safety population)
FDA Review
Figure 4 summarizes the percentage of patients by ethnicity.
Figure 4. Baseline Demographics by Ethnicity (safety population)
FDA Review
Who participated in the trials?
The table below summarizes demographics of patients from both trials who were dosed at recomended 80mg/kg/week.clinical trials.
Table 3. Baseline Demographics of Patients in the Clinicals
| Demographic Parameter | VILTEPSO (safety population) N=16 |
VILTEPSO (efficacy population) N=8 |
|---|---|---|
| Sex, n (%) | ||
| Male | 16 (100) | 8 (100) |
| Race, n (%) | ||
| White | 7 (43.8) | 7 (87.5) |
| Asian | 9 (56.3) | 1 (12.5) |
| Age (years) | ||
| Mean (SD) | 7.5 (2.3) | 7 (2) |
| Median | 7.5 | 7 |
| Minimum, maximum | 4, 12 | 5, 10 |
| Age group, n (%) | ||
| 4 to <6 years | 4 (25.0) | 1 (12.5) |
| 6 to <12 years | 11 (68.8) | 6 (75) |
| 12 to 17 years | 1 (6.3) | 1 (12.5) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 1 (6.3) | 1 (12.5) |
| Not Hispanic or Latino | 6 (37.5) | 6 (75) |
| Not reported | 9 (56.3) | 1 (12.5) |
| Country, n (%) | ||
| US | 6 (37.5) | 0 |
| Canada | 2 (12.5) | 0 |
| Japan | 8 (50) | 8 (100) |
FDA Review
How were the trials designed?
There were two trials that evaluated VILTEPSO for DMD. Data from Trial 1 were used to evaluate the benefits and side effects of VILTEPSO and data from Trial 2 to evaluate side effects only. All patients in both trials were on a stable dose of corticosteroids for at least 3 months before entering the trials.
In Trial 1, patients were randomly assigned to receive either VILTEPSO or placebo once a week for the first four weeks. Neither the patients nor the health care providers knew which treatment was being given. After the first 4 weeks all patients received VILTEPSO at two different strengths for an additional 20 weeks. The benefit was evaluated by measuring the level of dystrophin in muscle biopsies before the treatment and at week 25.
In Trial 2, patients received VILTEPSO at two different strengths once a week for 24 weeks. Collected data was used for side effects assessment.
How were the trials designed?
VILTEPSO for the treatment of DMD was evaluated in two clinical trials. Enrolled patients had a genotypically confirmed DMD diagnosis (a confirmed mutation of the DMD gene that is amenable to exon 53 skipping) and were on a stable dose of corticosteroids for at least 3 months prior to enrollment.
Trial 1 was a multicenter, 2 period, dose-finding trial. During the initial, double blind period (first 4 weeks), which evaluated the acute safety of VILTEPSO, patients were randomized to VILTEPSO or placebo. All patients were then re-randomized and received 20 weeks of open-label VILTEPSO 40 mg/kg once weekly (0.5 times the recommended dosage) or 80 mg/kg once weekly. The primary efficacy endpoint was the change from baseline in the dystrophin protein level (measured as % of normal) at Week 25.
Trial 2 was a multicenter, parallel-group, open-label, dose-finding trial. Patients were assigned to receive intravenous VILTEPSO 40 mg/kg once weekly (0.5 times the recommended dose) (N=8) or 80 mg/kg once weekly (N=8) for 24 weeks.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION