Drug Trials Snapshots: UPLIZNA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the UPLIZNA Package Insert for complete information.
UPLIZNA (inebilizumab-cdon)
up liz' nah
Viela Bio, Inc.
Approval date: June 11, 2020
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
UPLIZNA is used to treat neuromyelitis optica spectrum disorder (NMOSD) in adult patients with a particular antibody (who are anti-aquaporin-4 or AQP4 antibody positive).
NMOSD is a rare disease that most commonly affects the optic nerves and spinal cord. It is characterized by relapses (attacks) that cause vision loss and serious disability which can lead to death.
How is this drug used?
UPLIZNA is given directly into the vein (intravenous infusion) over 90 minutes by a healthcare provider. First two infusions are given two weeks apart followed by one infusion every six months.
What are the benefits of this drug?
UPLIZNA reduces attacks of the disease.
The risk of an NMOSD relapse in the patients who were treated with UPLIZNA was reduced by 77% when compared to the placebo treatment group.
What are the benefits of this drug (results of trials used to assess efficacy)?
The efficacy endpoint for UPLIZNA was the time to the first adjudicated relapse on or before Day 197 by a blinded, independent, adjudication committee, who determined whether the attack met protocol-defined criteria.
Table 1. Efficacy Results in Trial 1 in anti-AQP4 Antibody Positive NMOSD Patients
| Treatment Group | ||
|---|---|---|
| UPLIZNA N = 161 |
Placebo N = 52 |
|
| Time to Adjudication Committee-Determined Relapse (Primary Efficacy Endpoint) | ||
| Number (%) of patients with relapse | 18 (11.2%) | 22 (42.3%) |
| Hazard ratio (95% CI)a | 0.227 (0.121, 0.423) | |
| p-valuea | < 0.0001 | |
a Cox regression method, with placebo as the reference group.
UPLIZNA Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: The majority of patients in the clinical trial were women. Differences in response to UPLIZNA between men and women could not be determined.
- Race: The majority of patients in the clinical trial were White. Differences in response to UPLIZNA among races could not be determined.
- Age: The majority of patients in the clinical trial were younger than 65 years of age. Differences in response to UPLIZNA between patients below and above 65 years of age could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Subgroup efficacy analyses based on primary endpoint are presented below.
Table 2. Subgroup Analyses of Time to AC-determined NMOSD Attack
| Demographic Characteristic | Patients with Attack | Hazard Ratio (95% CI)* | |
|---|---|---|---|
| UPLIZNA N = 161 |
Placebo N = 52 |
||
| Sex, x/n (%) | |||
| Women | 16/151 (10.6) | 20/49 (40.8) | 0.225 (0.116, 0.435) |
| Men | 2/10 (20.0) | 2/3 (66.7) | 0.160 (0.022, 1.195) |
| Race, x/n (%) | |||
| American Indian or Alaskan Native | 3/11 (27.3) | 2/5 (40.0) | 0.532 (0.088, 3.213) |
| Asian | 6/37 (16.2) | 5/8 (62.5) | 0.198 (0.060, 0.653) |
| Black or African American | 1/14 (7.1) | 1/5 (20.0) | 0.331 (0.021, 5.310) |
| White | 8/86 (9.3) | 10/24 (41.7) | 0.189 (0.074, 0.480) |
| Other | 0/13 (0.0) | 4/10 (40.0) | NA |
| Age Group, x/n (%) | |||
| < 65 years | 16/155 (10.3) | 21/48 (43.8) | 0.199 (0.104, 0.382) |
| ≥65 years | 2/6 (33.3) | 1/4 (25.0) | 1.371 (0.124, 15.179) |
| Region, x/n (%) | |||
| US | 2/27 (7.4) | 3/11 (27.3) | 0.226 (0.038, 1.360) |
| All other countries | 16/134 (11.9) | 19/41 (46.3) | 0.224 (0.115, 0.435) |
x/n=number of patients with AC/number of patients in the subgroup
FDA Statistical Review
What are the possible side effects?
UPLIZNA may cause serious side effects including infusion reactions, infections, and a harm to un unborn baby.
The most common side effects are urinary tract infection and joint pain.
What are the possible side effects (results of trials used to assess safety)?
The table below lists adverse reactions that occurred in at least 2% of patients treated with UPLINZA and at greater incidence than in patients who received placebo.
Table 3. Adverse Reactions in Patients with NMOSD with an Incidence of at Least 5% with UPLIZNA and a Greater Incidence than Placebo
| Adverse Reactions | UPLIZNA N = 161 % |
Placebo N = 52 % |
|---|---|---|
| Urinary tract infection | 11 | 10 |
| Arthralgia | 10 | 4 |
| Headache | 8 | 8 |
| Back pain | 7 | 4 |
UPLIZNA Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The majority patients in the clinical trial were women. Differences in side effects between men and women could not be determined.
- Race: The majority patients in the clinical trial were White. Differences in side effects among races could not be determined.
- Age: The majority of patients in the clinical trial were younger than 65 years of age. Differences in side effects between patients below and above 65 years of age could not be determined.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The tables below list adverse reactions by sex, and race subgroups. Age was not anayzed because of small number of patients who were older than 65 years.
Table 4. Analyses of Adverse Events by Sex
| Sex | ||||
|---|---|---|---|---|
| Men | Women | |||
| UPLIZNA N=10 |
Placebo N=3 |
UPLIZNA N=151 |
Placebo N=49 |
|
| Any Adverse Event | 90% | 100% | 72% | 69% |
| UTI | 10% | 0% | 11% | 10% |
| Arthralgia | 10% | 0% | 10% | 4% |
Table 5. Analyses of Adverse Events by Race
| Race | ||||||||
|---|---|---|---|---|---|---|---|---|
| White | Asian | Black or AA | American Indian | |||||
| UPLIZNA N=86 |
Placebo N=24 |
UPLIZNA N=37 |
Placebo N=8 |
UPLIZNA N=14 |
Placebo N=5 |
UPLIZNA N=11 |
Placebo N=5 |
|
| Any Adverse Event | 72% | 71% | 87% | 63% | 71% | 80% | 27% | 40% |
| UTI | 15% | 8% | 3% | 13% | 14% | 20% | 18 | 0% |
| Arthralgia | 6% | 0% | 22% | 0% | 0% | 20% | 0% | 0% |
Clinical Trial Data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved UPLIZNA based on evidence from one clinical trial (Trial 1/ NCT02200770) of 213 patients with NMOSD. The trial was conducted at 82 sites in 24 countries (including the Unites States) in North and South America, Europe, Africa, Asia and Australia.
Figure 1 summarizes how many men and women were in the clinical trial.
Figure 1. Demographics by Sex
FDA Review
Figure 2 summarizes the percentage of patients by race in the clinical trial.
Figure 2. Demographics by Race
FDA Review
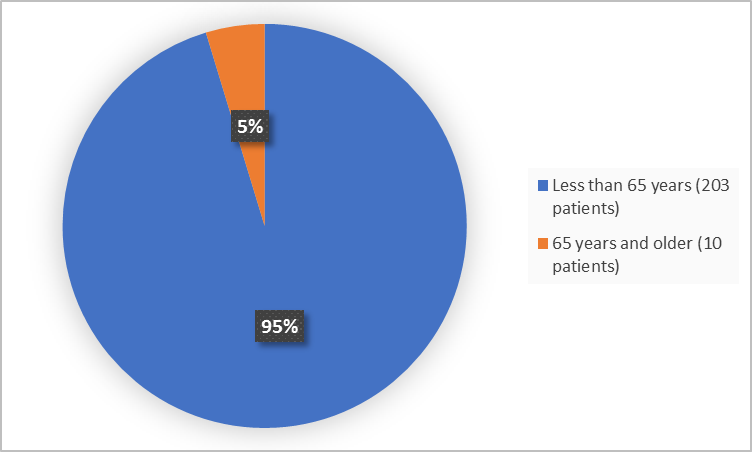
Figure 3 summarizes the percentage of patients by age in the clinical trial.
Figure 3. Demographics by Age
FDA Review
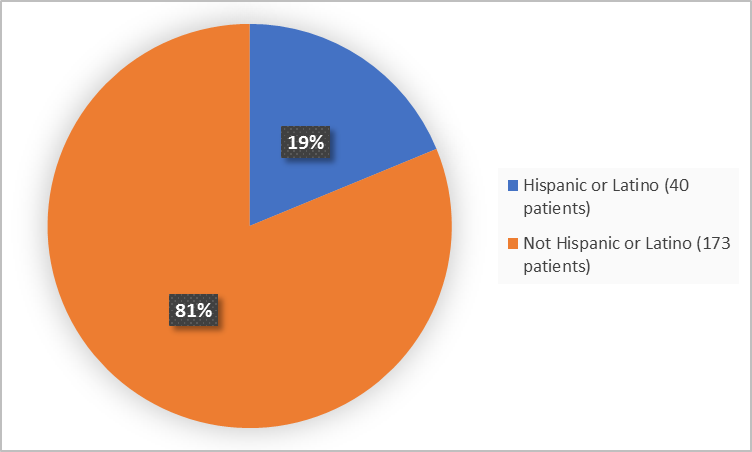
Figure 4 summarizes the percentage of patients by ethnicity in the clinical trial.
Figure 4. Demographics by Ethnicity
FDA Review
Who participated in the trials?
Demographics of the population that participated in the UPLIZNA trial are presented in the table below.
Table 6. Trial Demographics
| Demographic Characteristic | UPLIZNA N = 161 |
Placebo N = 52 |
Total N=213 |
|---|---|---|---|
| Sex, n(%) | |||
| Men | 10 (6.2) | 3 (5.8) | 13 (6.1) |
| Women | 151 (93.8) | 49 (94.2) | 200 (93.9) |
| Race, n(%) | |||
| American Indian or Alaskan Native | 11 (6.8) | 5 (9.6) | 16 (7.5) |
| Asian | 37 (23.0) | 8 (15.4) | 45 (21.1) |
| Black or African American | 14 (8.7) | 5 (9.6) | 19 (8.9) |
| White | 86 (53.4) | 24 (46.2) | 110 (51.6) |
| Other/Multiple | 13 (8.1) | 10 (19.2) | 23 (10.8) |
| Age (years) | |||
| Mean (SD) | 43.2 (11.6) | 42.4 (14.3) | 43 (12.3) |
| Median (min. max) | 43 (18, 73) | 43 (18,74) | 43 (18, 74) |
| Age Group n(%) | |||
| < 65 years | 155 (96.3) | 48 (92.3) | 203 (95.3) |
| ≥65 years | 6 (3.7) | 4 (7.7) | 10 (4.7) |
| Ethnicity, n(%) | |||
| Hispanic or Latino | 25 (15.5) | 15 (28.8) | 40 (18.8) |
| Not Hispanic or Latino | 136 (84.5) | 37 (71.2) | 173 (81.2) |
| Region n(%) | |||
| Africa | 1 (0.6) | 3 (5.8) | 4 (1.9) |
| Asia | 43 (26.7) | 7 (13.5) | 50 (23.5) |
| Canada | 2 (1.2) | 0 (0.0) | 2 (0.9) |
| Europe | 66 (41.0) | 16 (30.8) | 82 (38.5) |
| Other | 0 (0.0) | 2 (3.8) | 2 (0.9) |
| South America | 22 (13.7) | 13 (25.0) | 35 (16.4) |
| United States | 27 (16.8) | 11 (21.2) | 38 (17.8) |
FDA Review
How were the trials designed?
The safety and efficacy of UPLIZNA were established in a randomized (3:1) double-blind, placebo-controlled trial that enrolled 213 patients with NMOSD who were anti-AQP4 antibody positive.
The primary efficacy endpoint was the time to the onset of the first adjudicated relapse on or before Study Day 197 evaluated by a blinded, independent, adjudication committee, who determined whether the attack met protocol-defined criteria.
How were the trials designed?
There was one trial of 197 days duration that evaluated the benefits and side effects of UPLIZNA. The trial enrolled adult patients with NMOSD who were positive for anti-aquaporin-4 antibody. Patients received at random either UPLIZNA or placebo infusions according to the schedule. Neither the patients nor the healthcare providers knew which treatment was being given.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION