Drug Trials Snapshots: TIBSOVO
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to TIBSOVO Prescribing Information for complete information.
TIBSOVO (ivosidenib)
tib-SOH-voh
Agios Pharmaceuticals
Approval date: July 20, 2018
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
TIBSOVO is used to treat adults with acute myeloid leukemia (AML) that have a mutation in a gene called IDH1 and whose disease has come back or has not improved after previous treatment(s).
AML is a rapidly progressing cancer that forms in the bone marrow and can result in an increased number of white blood cells in the bloodstream.
How is this drug used?
TIBSOVO is a tablet. Two tablets (total of 500 mg) are taken once a day.
What are the benefits of this drug?
Of the 174 patients who received TIBSOVO, 33 percent experienced no evidence of disease and full recovery of blood counts (complete remission) or no evidence of disease and partial recovery of blood counts after treatment, which lasted about 8 months.
Of the 110 patients who required transfusions of blood or platelets due to AML at the start of the trial, 37 percent went at least 56 days without requiring a transfusion after treatment with TIBSOVO.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes efficacy results established on the basis of the rate of complete response (CR)/complete response with partial hematologic recovery (CRh), the duration of CR/CRh, and the rate of conversion from transfusion dependence to transfusion independence.
Table 2: Efficacy Results in Patients with Relapsed or Refractory Acute Myeloid Leukemia (AML)
| Endpoint | TIBSOVO (500 mg daily) N=174 |
|---|---|
| CR1 n (%) | 43 (24.7) |
| 95% CI | (18.5, 31.8) |
| Median DOR2 (months) | 10.1 |
| 95% CI | (6.5, 22.2) |
| CRh3 n (%) | 14 (8.0) |
| 95% CI | (4.5, 13.1) |
| Median DOR (months) | 3.6 |
| 95% CI | (1, 5.5) |
| CR+CRh4 n (%) | 57 (32.8) |
| 95% CI | (25.8, 40.3) |
| Median DOR (months) | 8.2 |
| 95% CI | (5.6, 12) |
CI: confidence interval
1 CR (complete remission) was defined as 100,000/microliter and absolute neutrophil counts [ANC] >1,000/microliter).
2 DOR (duration of response) was defined as time since first response of CR or CRh to relapse or death, whichever is earlier.
3 CRh (complete remission with partial hematological recovery) was defined as 50,000/microliter and ANC >500/microliter).
4 CR+CRh rate appeared to be consistent across all baseline demographic and baseline disease characteristics with the exception of number of prior regimens.
5%>5%>
TIBSOVO Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: TIBSOVO worked similarly in men and women.
- Race: Most of the patients were White. Differences in how well the drug worked among races could not be determined because of the small number of patients in other races.
- Age: TIBSOVO worked similarly in patients younger and older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The table below summarizes exploratory efficacy results by demographic subgroups.
Figure 4. Subgroup Analyses of Response Rate (CR+CRh ) by Sex, Race and Age
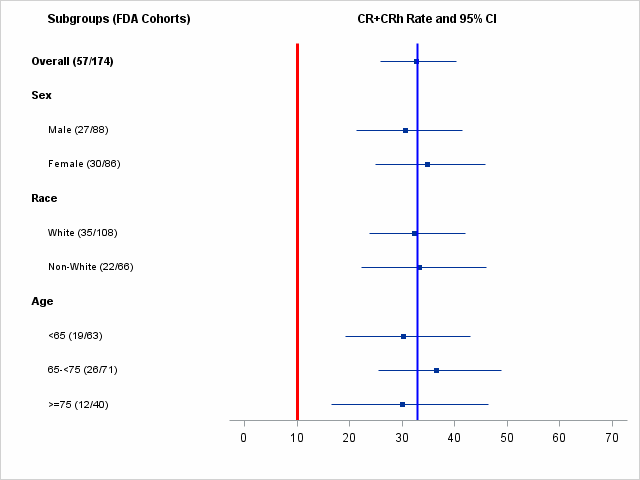
Adapted from FDA review
What are the possible side effects?
TIBSOVO may cause a serious and life threating side effect called differentiation syndrome. Other serious side effects include changes in the electrical activity of heart (QTc prolongation) and a nervous system problem called Guillain-Barré syndrome.
Common side effects of TIBSOVO are fatigue, increased white cell blood count, joint pain, diarrhea, shortness of breath, body swelling and nausea.
What are the possible side effects (results of trials used to assess safety)?
The tables below summarize adverse reactions and laboratory abnormalities in the clinical trial.
Table 3. Adverse Reactions Reported in ≥10% (Any Grade) or ≥5% (Grade ≥3) of Patients with Relapsed or Refractory AML
| TIBSOVO (500 mg daily) N=179 |
||
|---|---|---|
| Body System Adverse Reaction |
All Grades n (%) |
≥ Grade 3 n (%) |
| Blood System and Lymphatic System Disorders | ||
| Leukocytosis1 | 68 (38) | 15 (8) |
| Differentiation Syndrome2 | 34 (19) | 23 (13) |
| Gastrointestinal Disorders | ||
| Diarrhea | 60 (34) | 4 (2) |
| Nausea | 56 (31) | 1 (1) |
| Mucositis3 | 51 (28) | 6 (3) |
| Constipation | 35 (20) | 1 (1) |
| Vomiting4 | 32 (18) | 2 (1) |
| Abdominal pain5 | 29 (16) | 2 (1) |
| General Disorders and Administration Site Conditions | ||
| Fatigue6 | 69 (39) | 6 (3) |
| Edema7 | 57 (32) | 2 (1) |
| Pyrexia | 41 (23) | 2 (1) |
| Chest pain8 | 29 (16) | 5 (3) |
| Investigations | ||
| Electrocardiogram QT prolonged | 46 (26) | 18 (10) |
| Metabolism and Nutrition Disorders | ||
| Decreased appetite | 33 (18) | 3 (2) |
| Tumor lysis syndrome | 14 (8) | 11 (6) |
| Musculoskeletal and Connective Tissue Disorders | ||
| Arthralgia9 | 64 (36) | 8 (4) |
| Myalgia10 | 33 (18) | 1 (1) |
| Nervous System Disorders | ||
| Headache | 28 (16) | 0 |
| Neuropathy11 | 21 (12) | 2 (1) |
| Respiratory, Thoracic and Mediastinal Disorders | ||
| Cough12 | 40 (22) | 1 ( |
| Dyspnea13 | 59 (33) | 16 (9) |
| Pleural effusion | 23 (13) | 5 (3) |
| Skin and Subcutaneous Tissue Disorders | ||
| Rash14 | 46 (26) | 4 (2) |
| Vascular Disorders | ||
| Hypotension15 | 22 (12) | 7 (4) |
1 Grouped term includes leukocytosis, hyperleukocytosis, and increased white blood cell count.
2 Differentiation syndrome can be associated with other commonly reported events such as peripheral edema, leukocytosis, pyrexia, dyspnea, pleural effusion, hypotension, hypoxia, pulmonary edema, pneumonia, pericardial effusion, rash, fluid overload, tumor lysis syndrome, and creatinine increased.
3 Grouped term includes aphthous ulcer, esophageal pain, esophagitis, gingival pain, gingivitis, mouth ulceration, mucosal inflammation, oral pain, oropharyngeal pain, proctalgia, and stomatitis.
4 Grouped term includes vomiting and retching.
5 Grouped term includes abdominal pain, upper abdominal pain, abdominal discomfort, and abdominal tenderness.
6 Grouped term includes asthenia and fatigue.
7 Grouped term includes peripheral edema, edema, fluid overload, fluid retention, and face edema.
8 Grouped term includes angina pectoris, chest pain, chest discomfort, and non-cardiac chest pain.
9 Grouped term includes arthralgia, back pain, musculoskeletal stiffness, neck pain, and pain in extremity.
10 Grouped term includes myalgia, muscular weakness, musculoskeletal pain, musculoskeletal chest pain, musculoskeletal discomfort, and myalgia intercostal.
11 Grouped term includes ataxia, burning sensation, gait disturbance, Guillain-Barré syndrome, neuropathy peripheral, paresthesia, peripheral sensory neuropathy, peripheral motor neuropathy, and sensory disturbance.
12 Grouped term includes cough, productive cough, and upper airway cough syndrome.
13 Grouped term includes dyspnea, respiratory failure, hypoxia, and dyspnea exertional.
14 Grouped term includes dermatitis acneiform, dermatitis, rash, rash maculo-papular, urticaria, rash erythematous, rash macular, rash pruritic, rash generalized, rash papular, skin exfoliation, and skin ulcer.
15 Grouped term includes hypotension and orthostatic hypotension.
TIBSOVO Prescribing Information
Table 4. Most Common (≥10%) or ≥5% (Grade ≥3) New or Worsening Laboratory Abnormalities Reported in Patients with Relapsed or Refractory AML
| TIBSOVO (500 mg daily) N=179 |
||
|---|---|---|
| Parameter | All Grades n (%) |
≥ Grade 3 n (%) |
| Hemoglobin decreased | 108 (60) | 83 (46) |
| Sodium decreased | 69 (39) | 8 (4) |
| Magnesium decreased | 68 (38) | 0 |
| Uric acid increased | 57 (32) | 11 (6) |
| Potassium decreased | 55 (31) | 11 (6) |
| Alkaline phosphatase increased | 49 (27) | 1 (1) |
| Aspartate aminotransferase increased | 49 (27) | 1 (1) |
| Phosphate decreased | 45 (25) | 15 (8) |
| Creatinine increased | 42 (23) | 2 (1) |
| Alanine aminotransferase increased | 26 (15) | 2 (1) |
| Bilirubin increased | 28 (16) | 1 (1) |
TIBSOVO Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: Most of the patients were White. Differences in the occurrence of side effects among races could not be determined because of the small number of patients in other races.
- Age: The occurrence of side effects was similar in patients younger and older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Tables below summarize ≥ grade adverse events during the clinical trials by sex, race and age subgroup.
Table 5. Subgroup Analyses of Adverse Events
| Demographic Characteristic | TIBSOVO (N=179) Grade ≥ 3 TEAE |
|---|---|
| All Patients | 142/179 (79) |
| Sex, n/N (%) | |
| Men | 69/90 (77) |
| Women | 73/89 (82) |
| Race, n/N (%) | |
| White | 88/112 (79) |
| All Other/Not provided/Unknown | 54/67 (81) |
| Age Group, n/N (%) | |
| 65> | 58/67 (87) |
| >=65 years | 84/112 (75) |
TEAE-treatment-emergent adverse event
Adapted from Clinical trial report
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved TIBSOVO based on evidence from one clinical trial (NCT02074839) of 179 patients with AML whose disease has come back or has not improved after previous treatment(s). A total of 174 patients had a certain type of mutation (IDH1-mutation) which was confirmed using an FDA-approved test.
The trial was conducted in United States and France.
Figure 1 summarizes how many men and women were in the clinical trial.
Figure 1. Baseline Demographics by Sex
FDA review
Figure 2 and Table 1 below summarize the percentage of patients by race in the clinical trial.
Figure 2. Baseline Demographics by Race
FDA review
Table 1. Baseline Demographics by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 112 | 63 |
| Black or African American | 10 | 6 |
| Asian | 6 | 3 |
| Other | 9 | 5 |
| Not reported or Unknown* | 42 | 23 |
* collection of data not allowed by authority
FDA review
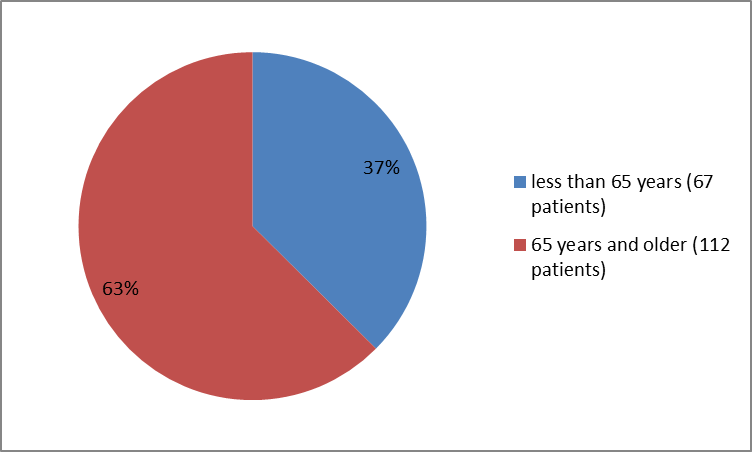
Figure 3 summarizes how many patients of a certain age were enrolled in the clinical trial.
Figure 3. Baseline Demographics by Age
FDA review
Who participated in the trials?
The table below summarizes demographics of all patients enrolled in the clinical trial who received TIBSOVO at recommended dose (safety population).
Table 6. Baseline Demographics of Patients Enrolled in the Clinical Trial (Safety population)
| Demographic Characteristic | TIBSOVO (n=179) |
|---|---|
| Sex | |
| Men | 90 (50%) |
| Women | 89 (50%) |
| Race | |
| White | 112 (63%) |
| Black | 10 (6%) |
| Asian | 6 (3%) |
| Other | 9 (5%) |
| Not Provided or Unknown* | 42 (23%) |
| Age (years) | |
| Mean | 65 |
| Median | 67 |
| Min, Max | 18, 87 |
| Age Group | |
| 18-65 years | 67 (37%) |
| ≥65 years | 112 (63%) |
| Ethnicity | |
| Not Hispanic or Latino | 100 (56%) |
| Hispanic or Latino | 9 (5%) |
| Not Provided* | 70 (39%) |
| Trial Site | |
| United States | 148 (83%) |
| France | 31 (17%) |
* collection of data not allowed by local authority
FDA Review
How were the trials designed?
There was one trial that evaluated the benefit and side effects of TIBSOVO in patients with AML whose disease has come back or has not improved after previous treatment(s). All patients had a certain type of mutation (IDH1-mutation) which was confirmed using an FDA-approved test.
Patients received TIBSOVO once a day until disease worsened or unacceptable toxicity.
The benefit of TIBSOVO was evaluated by measuring:
- how many patients reached complete remission (no evidence of disease) with full or partial recovery of blood counts after treatment,
- how long those patients remained in remission,
- and the percentage of patients who no longer required transfusions after treatment.
How were the trials designed?
The safety and efficacy of TIBSOVO were established in one open-label, single-arm, multicenter clinical trial of 179 adult patients with relapsed or refractory AML and an IDH1 mutation. Patients received TIBSOVO orally at dose of 500 mg daily until disease progression or unacceptable toxicity.
Efficacy was established on the basis of the rate of complete remission (CR) plus complete remission with partial hematologic recovery (CRh), the duration of CR+CRh, and the rate of conversion from transfusion dependence to transfusion independence.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION