Drug Trials Snapshots: OMEGAVEN
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the OMEGAVEN Package Insert for complete information.
OMEGAVEN (fish oil triglycerides)
o-mei-ge-ven
Fresenius Kabi USA, LLC
Approval date: July 27, 2018
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
OMEGAVEN is a drug that provides calories and fatty acids for pediatric patients with parenteral nutrition-associated cholestasis (PNAC).
Cholestasis is a condition in which bile is not released from the liver. It may occur when patients are receiving nutrition intravenously (parenteral nutrition).
How is this drug used?
OMEGAVEN is injected into a vein (intravenous) by a healthcare provider and is administered daily over 8 to 24 hours.
What are the benefits of this drug?
Patients treated with OMEGAVEN achieved age-appropriate weight.
What are the benefits of this drug (results of trials used to assess efficacy)?
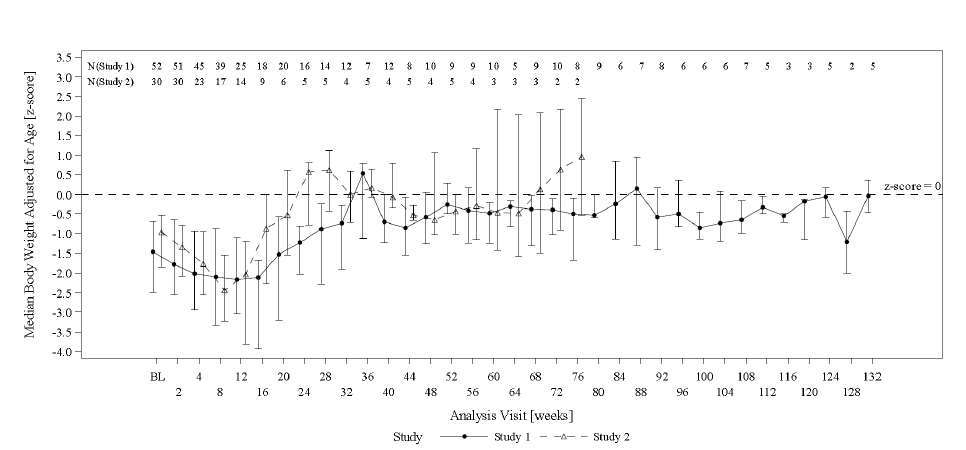
The figure below summarizes age-adjusted body weight over time for patients in the efficacy population in Trials 1 and 2. The main trial endpoint was the change in age-adjusted body weight (Z-scores) over time.
Figure 4. Median Age-Adjusted Body Weight (Z-Scores) Over time in OMEGAVEN-Treated Pediatric Patients with PNAC in Trial 1 and Trial 2
BL = baseline
Error bars represent interquartile ranges.
*Data from pair-matched OMEGAVEN patients were truncated at Week 132. Median values are only shown for visits with data from at least 2 patients at a particular visit.
Both trials measured direct bilirubin (DBil). At the end of the trials, the median DBil level for OMEGAVEN-treated patients was 0.60 mg/dL (interquartile range: 0.1 to 2.8 mg/dL). The Kaplan-Meier estimate of the median time for DBil values to return to less than 2.0 mg/dL was approximately 5.7 weeks.
OMEGAVEN Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
The trials that looked at the benefit of OMEGAVEN were too small to determine if there were any differences in sex, race, and age subgroups.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The trials that looked at the benefit of OMEGAVEN were too small to determine if there were any efficacy differences in sex, race, and age subgroups.
What are the possible side effects?
OMEGAVEN may cause serious side effects including death in preterm infants, severe allergic reactions, infection, fat overload syndrome, increased aluminum and lipid levels in the blood.
The most common side effects are vomiting, agitation, decreased heart rate, apnea (interruption of breathing), and viral infection.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions in patients with PNAC from both trials.
Table 2. Adverse Reactions in Greater Than 5% of OMEGAVEN-Treated Pediatric Patients with PNAC
| Adverse Reaction | OMEGAVEN (N=189) n (%) |
|---|---|
| Vomiting | 87 (46) |
| Agitation | 67 (35) |
| Bradycardia | 66 (35) |
| Apnea | 38 (20) |
| Viral Infection | 30 (16) |
| Erythema | 23 (12) |
| Rash | 15 (8) |
| Abscess | 14 (7) |
| Neutropenia | 13 (7) |
| Hypertonia | 11 (6) |
| Incision site erythema | 11 (6) |
OMEGAVEN Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar among males and females.
- Race: The occurrence of side effects was similar among White and Black or African American patients. The number of patients in other races was limited; therefore, differences in the occurrence of side effects among races could not be determined.
- Age: The majority of patients were less than 1 year of age; therefore, differences in side effects among age groups could not be determined.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes the occurrence of the most frequent adverse reaction, vomiting, by subgroup for the safety population in both trials.
Table 3. Subgroup Analysis of Vomiting (safety population)
| Demographic Characteristics | OMEGAVEN n/N (%) |
|---|---|
| Sex | |
| Male | 42/109 (39) |
| Female | 45/80 (56) |
| Race | |
| White | 59/119 (50) |
| Black or African American | 10/23 (43) |
| Asian | 4/7 (57) |
| Native Hawaiian or Other Pacific Islander | 0/1 (0) |
| Unknown | 14/39 (36) |
| Age Group | |
| 85/181 (47) | |
| > 52 weeks (> 1 year) | 2/8 (25) |
Clinical Trial Data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved OMEGAVEN based on evidence from two clinical trials (Trial 1/NCT00910104 and Trial 2/NCT00738101). The trials included 189 preterm, underweight, and/or severely ill pediatric patients with PNAC. Patients required prolonged intravenous administration of nutrients (parenteral nutrition) to assist with growth. The trials were conducted at three sites in the United States.
The safety population is presented below. Demographics of the efficacy population is presented in Table 5, under the MORE INFO section.
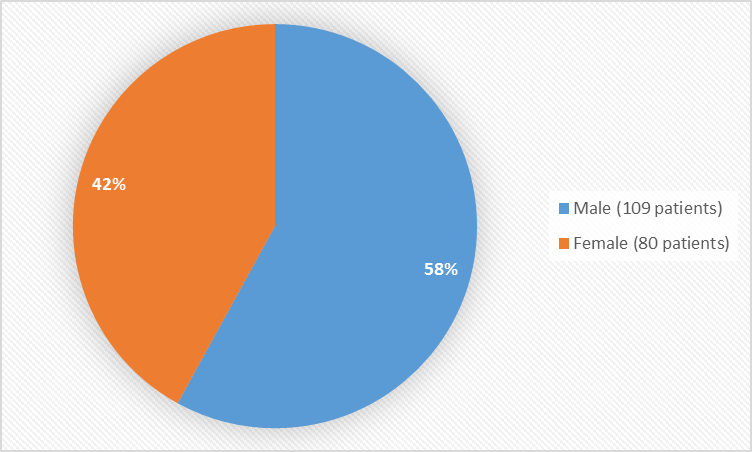
Figure 1 summarizes the percentage of patients by sex in the combined clinical trials to evaluate safety.
Figure 1. Baseline Demographics by Sex
FDA Review
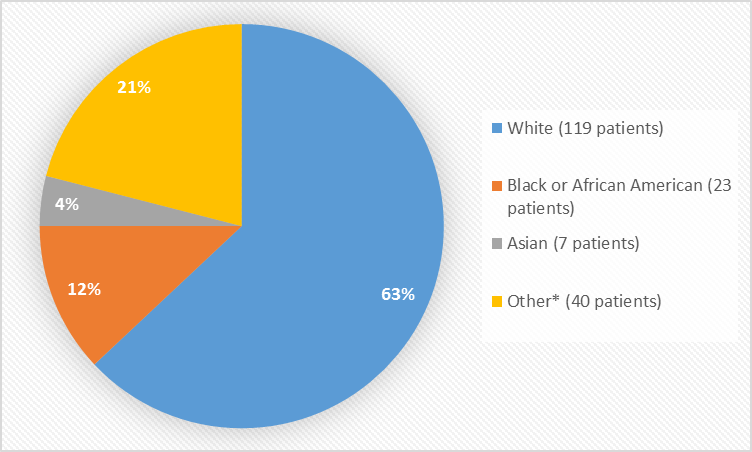
Figure 2 summarizes the percentage of patients by race in the combined clinical trials used to evaluate safety.
Figure 2. Baseline Demographics by Race
FDA Review
Table 1. Baseline Demographics by Race
| Race | Number of Patients | Percentage of Patients |
|---|---|---|
| White | 119 | 63% |
| Black or African American | 23 | 12% |
| Asian | 7 | 4% |
| Other* | 40 | 21% |
FDA Review
Figure 3 summarizes the percentage of patients by age in the combined clinical trials used to evaluate safety.
Figure 3. Baseline Demographics by Age
FDA Review
Who participated in the trials?
The table below summarizes demographics of all patients in the combined clinical trials.
Table 4. Demographic Characteristics for Trials 1 and 2 (safety population)
| Demographic Characteristic | OMEGAVEN N = 189 |
|---|---|
| Sex, n (%) | |
| Male | 109 (58) |
| Female | 80 (42) |
| Race, n (%) | |
| White | 119 (63) |
| Black or African American | 23 (12) |
| Asian | 7 (4) |
| Native Hawaiian or Other Pacific Islander | 1 (1) |
| Unknown | 39 (21) |
| Chronological Age at Baseline (Weeks) | |
| Mean (SD) | 24.5 (53.02) |
| Median | 10.3 |
| Range | 2 – 761 |
| Age Group in Weeks, n (%) | |
| 181 (96) | |
| > 52 weeks (> 1 year) | 8 (4) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 37 (20) |
| Not Hispanic or Latino | 31 (16) |
| Unknown | 121 (64) |
| Region | |
| United States | 189 (100) |
FDA Review
The table below summarizes demographics of all patients in the pair-matched efficacy population.
Table 5. Demographic Characteristics for the Pair-Matched Population (efficacy population)
| Demographic Characteristic | OMEGAVEN N = 82 | Historical Control N = 41 | Total N = 123 |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 42 (51) | 24 (59) | 66 (54) |
| Female | 40 (49) | 17 (41) | 57 (46) |
| Race, n (%) | |||
| White | 49 (60) | 27 (66) | 76 (62) |
| Black or African American | 11 (13) | 4 (10) | 15 (12) |
| Asian | 6 (7) | 1 (2) | 7 (6) |
| Native Hawaiian or Other Pacific Islander | 1 (1) | 0 (0) | 1 (less than 1) |
| Unknown | 15 (18) | 9 (22) | 24 (20) |
| Chronological Age at Baseline (Weeks) | |||
| Mean (SD) | 10.4 (6.82) | 7.9 (6.0) | 9.1 (6.2) |
| Median | 9.0 | 6.3 | 7.2 |
| Range | 3 – 42 | 0 – 41 | 0 - 42 |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 17 (20.7) | 14 (34.1) | 31 (25.2) |
| Not Hispanic or Latino | 15 (18.3) | 6 (14.6) | 21 (17.1) |
| Unknown | 50 (61.0) | 21 (51.2) | 71 (57.7) |
| Region | |||
| United States | 82 (100.0) | 41 (100.0) | 123 (100.0) |
FDA Review
How were the trials designed?
Trial 1 and Trial 2 were used to evaluate both, benefits and safety of OMEGAVEN. Both trials enrolled pediatric patients with PNAC. Trial 1 enrolled patients less than 2 years of age, and Trial 2 enrolled patients less than 5 years of age. Patients were treated once daily with OMEGAVEN for at least 14 days but no more than 5 months.
The benefit of OMEGAVEN was evaluated by measuring the body weight over time in OMEGAVEN treated patients and comparing it to similar patients who were treated in the past with soybean solution. Additional comparisons were made to age-standardized growth charts to assess age appropriate growth in enrolled patients treated with OMEGAVEN as their only lipid source.
How were the trials designed?
The efficacy and safety of OMEGAVEN were established in 2 open-label clinical trials in pediatric patients with PNAC who required parenteral nutrition for at least 14 days. Both trials enrolled preterm, underweight, or severely ill pediatric patients. Trial 1 enrolled patients less than 2 years of age. Trial 2 enrolled patients less than 5 years of age. Patients received OMEGAVEN (maximum dose of 1 g/kg/day) for no more than 5 months.
For efficacy analyses, historical pair-matched control patients who received a soybean oil-based lipid emulsion (maximum dose of 3 g/kg/day) were used as a comparator. The primary efficacy endpoint was the change over time in body weight adjusted for age. Additional comparisons were made to age-standardized growth charts to assess age appropriate growth in enrolled patients treated with OMEGAVEN as their only lipid source.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION