Drug Trials Snapshots: NOURIANZ
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the NOURIANZ Package Insert for complete information.
NOURIANZ (istradefylline)
Nue’-ree-anz
Kyowa Kirin, Inc.
Approval date: August 27, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
NOURIANZ is a drug used to treat “off” episodes in patients with Parkinson’s disease (PD). An “off” episode is a time when a patient’s medications are not working well, leading to an increase in Parkinson’s symptoms, such as tremor and difficulty walking.
How is this drug used?
NOURIANZ is a tablet taken by mouth once daily in addition to drugs for the treatment of Parkinson’s disease that contain levodopa/carbidopa combination.
What are the benefits of this drug?
Patients treated with NOURIANZ had decreased “off” time compared to patients treated with placebo.
What are the benefits of this drug (results of trials used to assess efficacy)?
The tables below summarize the efficacy results for individual trials based on the change in percentage of daily awake “off” time for NOURIANZ and placebo treated patients.
Table 2. Change from Baseline in Daily Awake OFF Time-Trials 1 and 2
| Baseline | Change from Baseline to Endpoint | |||
|---|---|---|---|---|
N |
(mean ± SD) % of awake “off” hours |
N |
(LSMD* vs. placebo), % awake “off” hours, (p-value) |
|
| Trial 1 | ||||
| Placebo | 66 | 37.2 ± 13.8 | 65 | -- |
| NOURIANZ 40 mg | 129 | 38.4 ± 16.2 | 126 | - 6.78 (p=0.007) |
| Trial 2 | ||||
| Placebo | 113 | 38.7 ± 11.6 | 113 | -- |
| NOURIANZ 20 mg | 112 | 39.8 ± 14.0 | 112 | - 4.57 (p=0.025) |
* LSMD: Least squares mean difference; a negative value indicates a greater reduction from baseline in “OFF” Time for NOURIANZ, relative to placebo.
SD: Standard Deviation
NOURIANZ Prescribing Information
Table 3. Change from Baseline in Daily OFF Time-Trials 3 and 4
| Baseline | Change from Baseline to Endpoint | |||
|---|---|---|---|---|
N |
(mean ± SD) hours |
N |
(LSMD* vs. placebo) hours ( p-value) |
|
| Trial 3 | ||||
| Placebo | 118 | 6.4 ± 2.7 | 118 | -- |
| NOURIANZ 20 mg | 115 | 6.8 ± 2.9 | 115 | -0.65 (p=0.028) |
| NOURIANZ 40 mg | 124 | 6.6 ± 2.5 | 124 | -0.92 (p=0.002) |
| Trial 4 | ||||
| Placebo | 123 | 6.3 ± 2.5 | 123 | -- |
| NOURIANZ 20 mg | 120 | 6.6 ± 2.7 | 120 | -0.76 (p=0.006) |
| NOURIANZ 40 mg | 123 | 6.0 ± 2.5 | 123 | -0.74 (p=0.008) |
* SMD: Least squares mean difference; a negative value indicates a greater reduction from baseline in “off” time for NOURIANZ, relative to placebo.
SD: Standard Deviation
NOURIANZ Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: NOURIANZ worked similarly in men and women.
- Race: Differences in how well the drug worked among races could not be determined.
- Age: NOURIANZ worked similarly in patients younger and older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The tables below summarize efficacy results by demographic subgroups.
Table 4. Subgroup Analysis of Change in Percentage of Awake Time per Day Spent in the OFF State from Baseline to Endpoint by Sex -Trials 1 and 2
| NOURIANZ LS Mean (SE)* |
Placebo LS Mean (SE) |
|||
|---|---|---|---|---|
| Trial 1 | ||||
| Men | -10.84 (1.75) | -3.37 (2.42) | ||
| Women | -10.15 (2.01) | -6.26 (2.84) | ||
| Trial 2 | ||||
| Men | -8.08 (1.73) | -6.17 (1.71) | ||
| Women | -11.17 (2.44) | -3.12 (2.47) | ||
*LS = least square; SE = standard error
FDA Review
The table below summarizes the efficacy results by sex for Trial 3 and Trial 4.
Table 5. Change from Baseline in Daily OFF Time by Sex-Trials 3 and 4
| Analysis Group | NOURIANZ 20mg/day Difference from Placebo Hours (95% CI) |
NOURIANZ 40mg/day Differences from Placebo Hours (95% CI) |
|---|---|---|
| Trial 3 | ||
| Men | -0.26 (-1.24, 0.71) | -0.32 (-1.29, 0.65) |
| Women | -0.82 (-1.60, -0.03) | -1.05 (-1.82, 0.28) |
| Trial 4 | ||
| Men | -0.41 (-1.52, 0.70) | -0.94 (-1.93, 0.05) |
| Women | -1.06 (-1.71, -0.42) | -0.97 (-1.69, -0.26) |
FDA Review
Table 6. Subgroup Analysis of Awake Time per Day Spent in OFF State from Baseline to Endpoint by Age Group-Trials 1 and 2
| Analysis Group | N | NOURIANZ 20 mg/day | NOURIANZ 40 mg/day |
|---|---|---|---|
| Placebo, NOURIANZ | Difference from Placebo Hours (95% CI) |
Difference from Placebo Hours (95% CI) |
|
| Trial 1 | |||
| Less than 65 years | 32, 71 | -10.72 (-17.30, -4.14) | |
| 65 years or older | 33, 55 | -1.99 (-8.95,4.98) | |
| Trial 2 | |||
| Less than 65 years | 64, 64 | -4.91 (-9.64, -0.19) | |
| 65 years or older | 49, 48 | -3.87 (-11.71, 3.98) | |
Table 7. Change from Baseline in Daily OFF Time by Age-Trials 3 and 4
| Analysis Group | NOURIANZ 20mg/day Difference from Placebo Hours (95% CI) |
NOURIANZ 40mg/day Differences from Placebo Hours (95% CI) |
|---|---|---|
| Trial 3 | ||
| Less than 65 years | -1.02 (-1.84, -0.20) | -0.69 (-1.46, 0.08) |
| 65 years or older | -0.42 (-1.25, 0.42) | -1.10 (-1.96, -0.23) |
| Trial 4 | ||
| Less than 65 years | -0.45 (-1.35, 0.46) | -0.24 (-1.17, 0.70) |
| 65 years or older | -0.85 (-1.62, -0.09) | -1.28 (-2.01, -0.54) |
FDA Review
Table 8. Subgroup Analysis of Change in Percentage of Awake Time per Day Spent in the OFF State from Baseline to Endpoint by Race-Trials 1 and 2
| Analysis Group | N | NOURIANZ 20 mg/day | NOURIANZ 40 mg/day |
|---|---|---|---|
| Placebo, NOURIANZ | Difference from Placebo Hours (95% CI) |
Difference from Placebo Hours (95% CI) |
|
| Trial 1 | |||
| White | 61, 121 | -6.01 (-10.67, -1.35) | |
| All Other | 4, 5 | - | |
| Trial 2 | |||
| White | 103, 108 | -4.25 (-8.36, -0.15) | |
| All Other | 10, 4 | -15.25 (-43.59, 13.08) | |
Subgroup analysis by race for Trials 3 and 4 was not conductedbecause all patients were Asian.
What are the possible side effects?
NOURIANZ can cause uncontrolled movement (dyskinesia), hallucinations, and unusual urges (impulse control or compulsive behaviors).
The most common side effects of NOURIANZ are uncontrolled movements, dizziness, constipation, nausea, hallucination, and insomnia.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions that occurred in the clinical trials.
Table 9. Adverse Reactions with an Incidence of at Least 2% in Patients Treated with NOURIANZ, and Greater than on Placebo, in Pooled Trials 1, 2, 3, and 4
| Adverse Reactions | NOURIANZ 20 mg/day (N=356) % |
NOURIANZ 40 mg/day (N=378) % |
Placebo N=426 % |
|---|---|---|---|
| Nervous system disorders Dyskinesia Dizziness |
15 3 |
17 6 |
8 4 |
| Gastrointestinal disorders Constipation Nausea Diarrhea |
5 4 1 |
6 6 2 |
3 5 1 |
| Psychiatric disorders Hallucination1 Insomnia |
2 1 |
6 6 |
3 4 |
| Metabolism and nutrition disorders Decreased appetite |
1 |
3 |
1 |
| Investigations Blood alkaline phosphatase increased Blood glucose increased Blood urea increased |
1 1 1 |
2 2 2 |
1 0 0 |
| Respiratory, thoracic and mediastinal disorders Upper Respiratory Tract Inflammation |
1 |
2 |
0 |
| Skin and subcutaneous tissue disorders Rash |
1 |
2 |
1 |
1 Includes hallucinations, hallucinations visual, hallucinations olfactory, hallucinations somatic, hallucinations auditory.
NOURIANZ Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects between men and women was similar.
- Race: The occurrence of side effects was similar between White and Asian patients.
- Age: The occurrence of side effects was similar between patients younger and older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The tables below summarize most common adverse reactions by sex, race, and age.
Table 10. Adverse Events by Sex
| NOURIANZ 20mg | NOURIANZ 40mg | Placebo | ||||
|---|---|---|---|---|---|---|
| Men N=168 n (%) |
Women N = 188 n (%) |
Men N = 197 n (%) |
Women N=181 n (%) |
Men N = 221 n (%) |
Women N = 205 n (%) |
|
| Dyskinesia | 20 (12) | 32 (17) | 25 (13) | 38 (15) | 12 (5) | 21 (10) |
| Constipation | 11 (7) | 8 (4) | 11 (6) | 10 (6) | 6 (3) | 7 (3) |
| Nausea | 10 (6) | 5 (3) | 13 (7) | 11 (6) | 9 (4) | 11 (5) |
| Dizziness | 2 (1) | 9 (5) | 12 (6) | 10 (6) | 8 (4) | 9 (4) |
| Hallucinations | 3 (2) | 5 (3) | 9 (5) | 12 (7) | 10 (5) | 1 (<1) |
| Insomnia | 1 (1) | 1 (1) | 14 (7) | 8 (4) | 9 (4) | 6 (3) |
Clinical Trial Data
Table 11. Adverse Events by Race
| NOURIANZ 20mg | NOURIANZ 40mg | Placebo | ||||
|---|---|---|---|---|---|---|
| White N = 106 n (%) |
Asian N = 242 n (%) |
White N = 124 n (%) |
Asian N = 250 n (%) |
White N = 166 n (%) |
Asian N = 251 n (%) |
|
| Dyskinesia | 26 (25) | 26 (11) | 38 (31) | 23 (9) | 23 (14) | 9 (4) |
| Nausea | 8 (8) | 6 (2) | 16 (13) | 7 (3) | 13 (8) | 7 (3) |
| Constipation | 5 (5) | 13 (5) | 9 (7) | 12 (5) | 2 (1) | 11 (4) |
| Dizziness | 9 (8) | 2 (1) | 18 (15) | 3 (1) | 12 (7) | 4 (2) |
| Hallucinations | 2 (2) | 6 (2) | 8 (6) | 13 (5) | 10 (6) | 1 (<1) |
| Insomnia | 1 (1) | 1 (<1) | 17 (14) | 4 (2) | 12 (7) | 3 (1) |
Clinical Trial Data
Table 12. Adverse Events by Age
| NOURIANZ 20mg | NOURIANZ 40mg | Placebo | ||||
|---|---|---|---|---|---|---|
| <65 N = 164 n (%) |
≥65 years N = 192 n (%) |
<65 N = 182 n (%) |
≥65 years N= 196 n (%) |
<65 N = 211 n (%) |
≥65 years N = 215 n (%) |
|
| Dyskinesia | 28 (17) | 24 (13) | 34 (19) | 29 (15) | 16 (8) | 17 (8) |
| Constipation | 11 (7) | 8 (4) | 8 (4) | 13 (7) | 6 (3) | 7 (3) |
| Nausea | 10 (6) | 5 (3) | 14 (8) | 10 (5) | 12 (6) | 8 (4) |
| Dizziness | 3 (2) | 8 (4) | 13 (7) | 9 (5) | 8 (4) | 9 (4) |
| Hallucinations | 2 (1) | 6 (3) | 7 (4) | 14 (7) | 5 (2) | 6 (3) |
| Insomnia | 2 (1) | 0 (0) | 15 (8) | 7 (4) | 9 (4) | 6 (3) |
Clinical Trial Data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved NOURIANZ based on evidence from 4 clinical trials (Trial 1/ NCT00456586, Trial 2/NCT00199407, Trial 3/NCT00455507, Trial 4/NCT00955526) of 1,160 patients with PD whose symptoms were not well controlled while receiving their regular PD treatment. Trial 1 was conducted in the US and Canada, Trial 2 in the US, and Trials 3 and 4 in Japan.
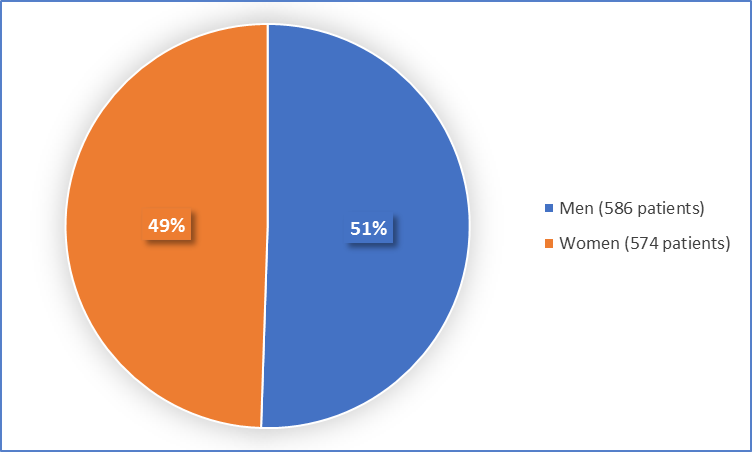
Figure 1 summarizes how many men and women were in the clinical trials.
Figure 1. Baseline Demographics by Sex
FDA Review
Figure 2 and Table 1 summarize the percentage of patients by race in the clinical trials.
Figure 2. Baseline Demographics by Race
FDA Review
Table 1. Demographics of Trials by Race
| Race | Number of Patients | Percentage of Patients |
|---|---|---|
| White | 400 | 34% |
| Black or African-American | 9 | 1% |
| Asian | 743 | 64% |
| Other | 8 | 1% |
FDA Review
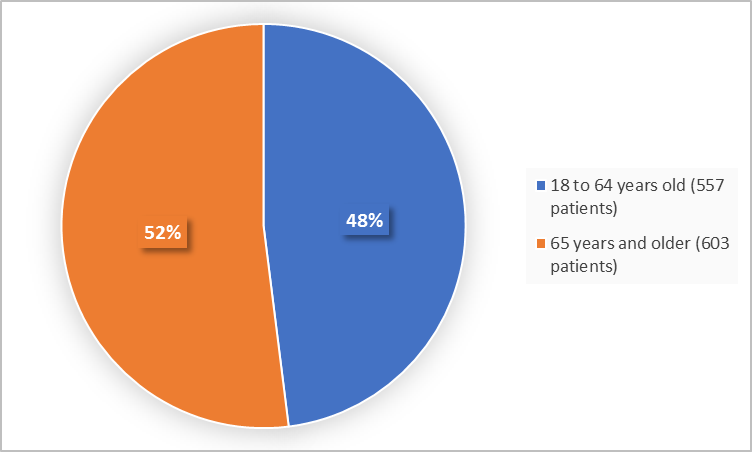
Figure 3 summarizes the percentage of patients by age group in the trials.
Figure 3. Baseline Demographics by Age
FDA Review
Who participated in the trials?
The table below summarizes the demographics for the combined four clinical trials.
Table 13. Demographics for the Trial Population
| Demographic Parameters | All NOURIANZ N = 734 n (%) |
Placebo N = 426 n (%) |
Total N = 1160 N (%) |
|---|---|---|---|
| Sex | |||
| Men | 365 (50) | 221 (52) | 586 (51) |
| Women | 369 (50) | 205 (48) | 574 (49) |
| Race | |||
| White | 233 (32) | 167 (39) | 400 (34) |
| Black or African American | 3 (<1) | 6 (1) | 9 (1) |
| Asian | 492 (67) | 251 (59) | 743 (64) |
| Other | 6 (1) | 2 (<1) | 8 (1) |
| Age Group | |||
| < 65 years | 346 (47) | 211 (50) | 557 (48) |
| ≥ 65 years | 388 (53) | 215 (50) | 603 (52) |
| Ethnicity | |||
| Not Collected | 734 (100) | 426 (100) | 1160 (100) |
| Region | |||
| United States | 232 (32) | 175 (41) | 407 (35) |
| Canada | 12 (2) | 6 (1) | 18 (2) |
| Japan | 490 (67) | 245 (58) | 735 (63) |
FDA Review
How were the trials designed?
There were four 12-week trials conducted in PD patients with inadequate control of their Parkinson’s symptom (“off” time) while receiving carbidopa/levodopa and other PD medications. Patients were randomly selected to receive either NOURIANZ or placebo pill once a day for 12 weeks. Neither the patients nor the health care providers knew which new treatment was being given until the trial was completed 12 weeks later.
In all trials, patients kept daily diaries of the number of hours they were awake and the number of hours of “off” time. The benefit was evaluated by measuring the change from baseline in total daily “off” time in NOURIANZ and placebo receiving patients.
How were the trials designed?
The safety and efficacy of NOURIANZ for the adjunctive treatment to levodopa/carbidopa patients with Parkinson’s disease experiencing “off” episodes was shown in four randomized, multicenter, double-blind, 12-week, placebo-controlled trials. Patients enrolled in the trials had been treated with levodopa for at least one year and were experiencing at least two hours of “off” time per day. Patients continued levodopa and other Parkinson’s Disease medications during the trials. The trials measured the change from baseline in the daily awake percentage of “off” time, or the change from baseline in total daily “off” time, based on 24-hour diaries completed by patients.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.