Drug Trials Snapshots: AKYNZEO
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the AKYNZEO Package Insert for complete information.
AKYNZEO (fosnetupitant and palonosetron)
(a kin zee oh)
HELSINN Healthcare SA
Approval date: April 19, 2018
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
AKYNZEO is an injectable drug for the prevention of the nausea and vomiting that happens right away or later in adults receiving certain anticancer medicines (chemotherapy).
AKYNZEO is a combination of two drugs, fosnetupitant and palonosetron. Fosnetupitant is a new drug never before approved by FDA. Palonosetron alone was previously approved by the FDA for the prevention of nausea and vomiting associated with cancer chemotherapy and surgery. Palonosetron is also a component of AKYNZEO capsules that was previously approved by the FDA for the prevention of nausea and vomiting associated with cancer chemotherapy.
How is this drug used?
AKYNZEO is given in combination with dexamethasone (steroid).
AKYNZEO is given by healthcare provider 30 minutes before the start of chemotherapy directly into the vein (IV infusion). It takes about 30 minutes to receive the full dose.
What are the benefits of this drug?
AKYNZEO prevents nausea and vomiting during the first 24 hours and up to 120 hours after chemotherapy.
What are the benefits of this drug (results of trials used to assess efficacy)?
Trial 1 was non-inferiority trial of palonosetron 0.25 mg administered as a 30-minute IV infusion versus a single dose of palonosetron 0.25 mg administered as a 30 second IV bolus. Primary endpoint was a complete response defined as no emetic episode and no rescue medication during the 0-24 hours after the start of chemotherapy. Previous findings of efficacy for IV palonosetron were also considered.
Results of the trial are shown in the table below.
Table 2. Complete Response in the Acute Phase, Trial 1 (Full Analysis Population)
|
IV Palonosetron 0.25 mg |
30-minute infusion |
30-second bolus |
Risk Difference** |
|---|---|---|---|
|
Acute Phase (24 h after highly emetogenic chemotherapy |
|||
|
Complete Response |
186 (82.7) |
186 (86.5) |
-3.8 [-12.2; 4.7] |
*: Wilson score method confidence interval
**: CMH stratum-adjusted method for difference in proportions, stratified by sex and country according to Koch et al. and O’Gorman et al. The non-inferiority margin is set to 15%.
Adapted from FDA Review
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: AKYNZEO worked similarly in men and women.
- Race: All patients in the trial were White. Differences in how the drug worked among races cannot be determined.
- Age: AKYNZEO worked similarly in patients younger or older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race and age groups?
All patients enrolled in Trial 1 were White. Subgroup analysis by sex and age are presented in the table below.
Table 3. Complete Response in the Acute Phase by Subgroup, Trial 1 (Full Analysis Population)
|
IV Palonosetron |
30-minute infusion |
30-second bolus |
|---|---|---|
|
Sex |
||
|
Men |
134/151 (88.7) |
129/144 (89.6) |
|
Women |
52/74 (70.3) |
57/71 (80.3) |
|
Age |
||
|
<65> |
123/156 (78.9) |
134/157 (85.4) |
|
≥65 years |
63/69 (91.3) |
52/58 (89.7) |
Adapted from FDA Review
What are the possible side effects?
AKYNZEO may cause serious allergic reaction, including hives, swollen face, difficulty breathing or chest pain.
AKYNZEO may cause a serious and potentially fatal reaction called serotonin syndrome. Symptoms of serotonin syndrome may include agitation, hallucinations or change in mental state, dizziness, fast heartbeat, sweating, low body temperature, shaking, muscle twitching, seizures, vomiting and diarrhea.
The most common side effects of injectable AKYNZEO are generally similar to those seen with AKYNZEO capsules which include headache, weakness, fatigue, heartburn, constipation and skin redness.
What are the possible side effects (results of trials used to assess safety)?
The safety of AKYNZEO for injection was evaluated in Trial 2 in patients receiving highly emetic chemotherapy by comparing it to AKYNZEO capsules. The safety profile of AKYNZEO for injection was generally similar to that seen with AKYNZEO capsules.
The treatment emergent adverse events that were noted in 2% or more of patients receiving AKYNZEO for injection are listed in the table below.
Table 4. Treatment-Emergent Adverse Events (TEAEs) Occurring in ≥2% of Patients Receiving AKYNZEO Injection or Capsule-Trial 2
|
|
IV AKYNZEO |
Oral Akynzeo |
|---|---|---|
|
Patients experiencing at least one TEAE |
170 (83.7) |
174 (86.6) |
|
Abdominal pain upper |
7 (3.4) |
7 (3.5) |
|
Alopecia |
32 (15.8) |
29 (14.4) |
|
ALT increased |
15 (7.4) |
24 (11.9) |
|
Anemia |
34 (16.7) |
39 (19.4) |
|
AST increased |
12 (5.9) |
19 (9.5) |
|
Asthenia |
15 (7.4) |
17 (8.5) |
|
Constipation |
21 (10.3) |
27 (13.4) |
|
Creatinine increased |
12 (5.9) |
6 (3) |
|
Diarrhea |
8 (3.9) |
10 (5.0) |
|
Fatigue |
18 (8.9) |
17 (8.5) |
|
Gastroesophageal reflux |
5 (2.5) |
0 |
|
Headache |
11 (5.4) |
9 (4.5) |
|
Hypertension |
8 (3.9) |
13 (6.5) |
|
Leukopenia |
21 (10.3) |
21 (10.5) |
|
Nausea |
20 (9.9) |
14 (7.0) |
|
Weight decreased |
9 (4.4) |
4 (2.0) |
|
Neutropenia |
61 (30.0) |
58 (28.9) |
Clinical Trial Data
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: Most of the patients in the trial were White. Differences in the occurrence of side effects among races could not be determined, because of the small number of patients in other races.
- Age: The occurrence of side effects was similar in patients younger or older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race and age groups?
More than 99% of patients in Trial 2 were White. The frequency of treatment-emergent adverse events by sex and age category are shown in the table below.
Table 5. Occurrence of Treatment Emergent Adverse Events by Subgroup – Trial 2
|
|
IV AKYNZEO |
Oral Akynzeo |
|---|---|---|
|
Sex |
||
|
Men |
86/107 (80.4) |
91/107 (85.0) |
|
Women |
84/96 (87.5) |
85/94 (90.4) |
|
Age |
||
|
< 65=""> |
108/129 (83.7) |
116/132 (87.9) |
|
≥65 years |
62/74 (83.7) |
60/69 (87.0) |
Clinical Trial Data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved injectable AKYNZEO based primarily on two trials (Trial 1 - NCT# 02557035 and Trial 2 - NCT # 02517021). FDA also considered data from previous trials used to approve AKYNZEO capsules and its components.
Trial 1 was used to establish the benefits of AKYNZEO and enrolled 440 patients receiving chemotherapy. This trial was conducted at 76 centers in 9 countries: Belarus, Bosnia and Herzegovina, Bulgaria, Georgia, Greece, Hungary, Lithuania, Romania, and Russia.
Trial 2 was used to establish side effects of AKYNZEO and included 404 patients (safety population) receiving chemotherapy and This trial was conducted at 80 centers in 11 countries: Austria, Croatia, Czech Republic, Germany, Israel, Italy, Poland, Serbia, Spain, Ukraine, US.
Figures 1 and 2 summarize how many men and women were enrolled in the clinical Trial 1 (efficacy population) and Trial 2 (safety population) respectively.
Figure 1. Baseline Demographics by Sex – Efficacy Population
Clinical Trial Data
Figure 2. Baseline Demographics by Sex – Safety Population
Clinical Trial Data
Figures 3 and 4 and Table 1 summarize the percentage of patients by race enrolled in the clinical Trial 1 (efficacy population) and Trial 2 (safety population), respectively.
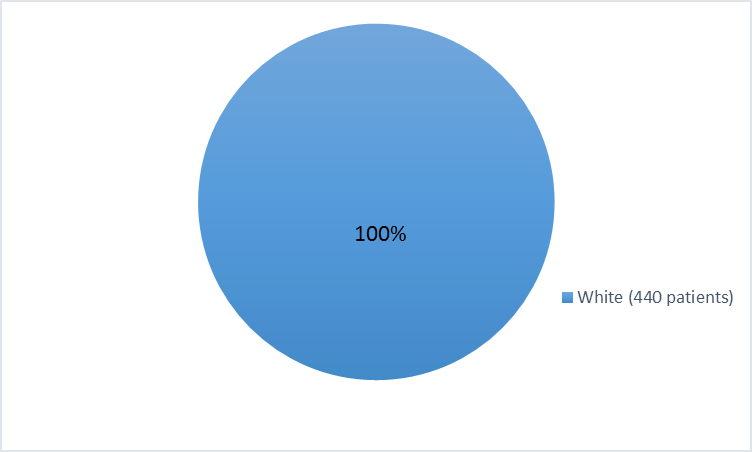
Figure 3. Baseline Demographics by Race – Efficacy Population
Clinical Trial Data
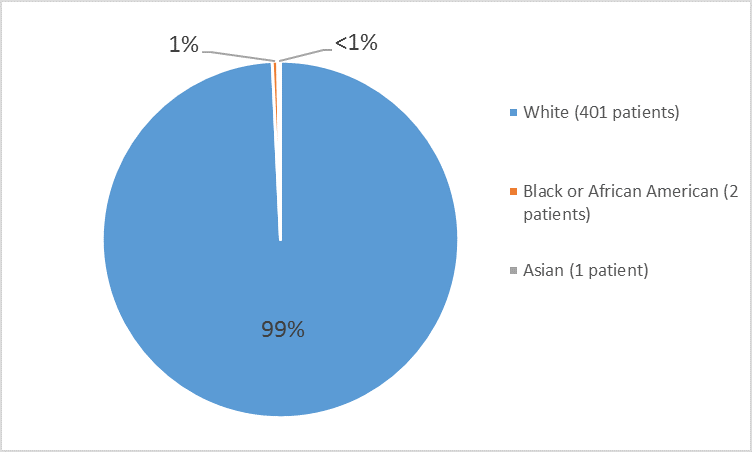
Figure 4. Baseline Demographics by Race – Safety Population
Clinical Trial Data
Table 1. Demographics of Trials by Race
|
|
Trial 1 |
Trial 2 |
||
|---|---|---|---|---|
|
Number of Patients |
Percentage |
Number of Patients |
Percentage |
|
|
White |
440 |
100 |
401 |
99 |
|
Black or African American |
0 |
0 |
2 |
less than 1 |
|
Asian |
0 |
0 |
1 |
less than 1 |
Clinical Trial Data
Figures 5 and 6 summarize the percentage of patients by age group enrolled in the clinical Trial 1 (efficacy population) and Trial 2 (safety population), respectively.
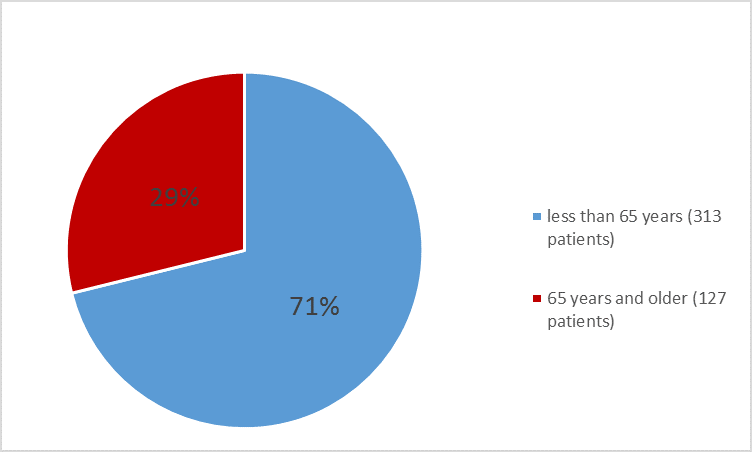
Figure 5. Baseline Demographics by Age – Efficacy Population
Clinical Trial Data
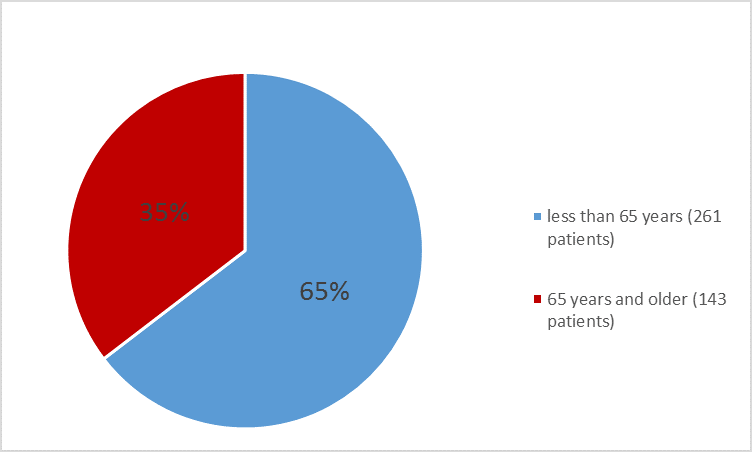
Figure 6. Baseline Demographics by Age – Safety Population
Clinical Trial Data
Who participated in the trials?
Table 6. Demographics of Trials 1 and 2
|
|
Trial 1 |
Trial 2 |
|---|---|---|
|
Sex |
||
|
Men |
295 (67.0) |
214 (53.0) |
|
Women |
145 (33.0) |
190 (47.0) |
|
Race |
||
|
White |
440 (100) |
401 (99.3) |
|
Black or African American |
0 |
2 (0.5) |
|
Asian |
0 |
1 (0.2) |
|
Age |
||
|
<65> |
313 (71.1) |
261 (64.6) |
|
≥65 years |
127 (28.9) |
143 (35.4) |
|
Ethnicity |
||
|
Hispanic or Latino |
Not Reported |
Not Reported |
|
Geographic Location |
||
|
United States |
0 |
12 (3.0) |
|
Rest of the World |
440 (100) |
392 (97.0) |
Clinical Trial data
How were the trials designed?
Trial 1 was used to assess the benefits of AKYNZEO. Patients who were receiving cancer chemotherapy that is known to result in nausea and vomiting were randomly assigned to receive palonosetron (one of the two drugs in AKYNZEO) by vein either as an injection (30 seconds duration) or as an infusion (over 30 minutes) in addition to dexamethasone. Both groups also received a placebo injection or infusion to make sure that neither the patient nor the healthcare provider knew which treatment was given. Patients in both groups were evaluated and compared for the occurrence of nausea and vomiting during 24-hours from the start of chemotherapy and up to 120 hours.
Trial 2 was used to assess the side effects of AKYNZEO. In this trial, patients receiving cancer chemotherapy that is known to result in nausea and vomiting were randomly assigned to receive either AKYNZEO capsules by mouth or AKYNZEO by injection 30 minutes before chemotherapy in addition to dexamethasone. Both groups also received a placebo injection or capsule to make sure that neither the patient nor the healthcare provider knew which treatment was given.
How were the trials designed?
AKYNZEO for injection was primarily approved based on two trials that enrolled patients who were receiving highly emetogenic chemotherapy. Previous findings of efficacy for IV palonosetron and of safety for AKYNZEO capsules were also considered.
Trial 1 was a multicenter, randomized, double-blind, double-dummy, non-inferiority trial comparing a 30-second bolus injection of IV palonosetron 0.25 mg to a 30-minute infusion of IV palonosetron 0.25 mg. All patients received dexamethasone before the start of chemotherapy. Primary endpoint was complete response defined as no emetic episode and no rescue medication during the 0-24 hours after the start of the chemotherapy. Complete response in the delayed phase (>24-120 hours after start of chemotherapy) was also reported.
Trial 2 was a multicenter, randomized, double-blind, double-dummy trial comparing the safety of AKYNZEO infusion to AKYNZEO capsules for the prevention of nausea and vomiting of highly emetogenic chemotherapy (including cisplatin, cyclophosphamide, carmustine, dacarbazine and mechloretamine) in four repeated consecutive chemotherapy cycles. All patients received dexamethasone before the start of chemotherapy.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.