Drug Trials Snapshots: ADLYXIN
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the ADLYXIN Prescribing Information for complete information.
ADLYXIN (lixisenatide)
ad-LIX-in
Sanofi-Aventis U.S. LLC
Approval date: July 27, 2016
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
ADLYXIN is a drug that improves blood sugar control in adults with diabetes mellitus (DM) type 2 when used in addition to diet and exercise.
How is this drug used?
ADLYXIN is available as a liquid that comes in a prefilled pen. It is injected once daily under the skin (subcutaneously) before the first meal of the day.
ADLYXIN may be used alone or in combination with other FDA-approved diabetic medications such as metformin, sulfonylureas, pioglitazone and insulin.
What are the benefits of this drug?
In patients with type 2 diabetes, treatment with ADLYXIN can lower HbA1c (hemoglobin A1c, which is a measure of blood sugar control).
What are the benefits of this drug (results of trials used to assess efficacy)?
The efficacy of ADLYXIN has been studied as monotherapy, in combination with oral antidiabetic medications, and in combination with basal insulin (with or without oral antidiabetic medications). The primary efficacy endpoint was the change in HbA1c from the start of each trial. Presented below are the results for each trial separately.
Table 3. Placebo Controlled Study (12 week Treatment Period Results) - Intent-To-Treat (ITT) Population
| Placebo (N=122) |
ADLYXIN 20 mcg (N= 119) |
|
|---|---|---|
| HbA 1c (%) | ||
| Baseline (mean) LS mean change from baseline# Difference from placebo (95% CI) |
8.07 -0.18 |
8.07 -0.83 -0.65 (-0.903, -0.399) (p<> |
| Patients (%) achieving HbA 1c <> | 24 | 44 |
| Fasting Plasma Glucose (FPG) (mg/dL) | ||
| Baseline (mean) LS mean change from baseline# |
160.39 1.46 |
162.77 -15.84 |
| Body weight (kg) | ||
| Baseline (mean) LS mean change from baseline# |
86.08 -2.03 |
86.50 -1.94 |
ITT population = all randomized patients.
# Using multiple imputation with respect to jump to placebo for missing data at Week 12 in the ADLYXIN group.
## Patients with missing HbA1c value at Week 12 were considered non-responders.
10% of patients in ADLYXIN and 10% in the placebo had missing HbA1c data at Week 12 in the ITT population.
ADLYXIN Prescribing Information
Table 4: Placebo Controlled Study in Patients with Type 2 Diabetes Mellitus in Combination with Metformin (24 week Results) - ITT Population
| Background therapy | With metformin* | |
|---|---|---|
| Placebo (N= 162) |
ADLYXIN 20 mcg (N= 161) |
|
| HbA 1c (%) | ||
| Baseline (mean) LS mean change from baseline# Difference from placebo (95% CI) |
8.03 -0.26 |
7.99 -0.72 -0.46 (-0.640, -0.279) (p<> |
| Patients (%) achieving HbA 1c<> | 22 | 44 |
| Fasting Plasma Glucose (FPG) (mg/dL) | ||
| Baseline (mean) LS mean change from baseline# Difference from placebo (95% CI) |
170.32 -7.25 |
172.23 -16.88 -9.64 (-16.306, -2.970) (p=0.0046) |
| Body weight (kg) | ||
| Baseline (mean) LS mean change from baseline# Difference from placebo (95% CI) |
87.87 -1.71 |
90.21 -2.70 -1.00 (-1.706, -0.286) (p=0.006) |
ITT population = all randomized patients.
# Using multiple imputation with respect to jump to placebo for missing data at Week 24 in the ADLYXIN group.
## Patients with missing HbA1c value at Week 24 were considered non-responders.
* 11% of patients in ADLYXIN 20 mcg and 6% in the placebo had missing HbA1c data at Week 24 in the ITT population
ADLYXIN Prescribing Information
Table 5: Placebo Controlled Study in Asian Patients with Type 2 Diabetes Mellitus in Combination with Metformin with or without Sulfonylurea (24 week Results) – ITT Population
| Background therapy | With metformin +/- sulfonylurea* | |
|---|---|---|
| Placebo (N= 195) |
ADLYXIN 20 mcg (N= 196) |
|
| HbA1c (%) | ||
| Baseline (mean) LS mean change from baseline# Difference from placebo (95% CI) |
7.85 -0.57 |
7.95 -0.84 -0.27 (-0.447, -0.090) (p=0.0032) |
| Patients (%) achieving HbA1c <> | 37 | 49 |
| Fasting Plasma Glucose (FPG) (mg/dL) | ||
| Baseline (mean) LS mean change from baseline# |
157.47 -7.05 |
159.26 -13.39 |
| Body weight (kg) | ||
| Baseline (mean) LS mean change from baseline# |
72.74 -1.12 |
73.18 -1.36 |
ITT population = all randomized patients.
# Using multiple imputation with respect to jump to placebo for missing data at Week 24 in the ADLYXIN group.
## Patients with missing HbA1c value at Week 24 were considered non-responders.
* 7% of patients in ADLYXIN and 6% in the placebo had missing HbA1c data at Week 24 in the ITT population.
ADLYXIN Prescribing Information
Table 6: Active Controlled Study in Patients with Type 2 Diabetes Mellitus in Combination with Metformin (24 week Treatment Period Results) - ITT population
| ADLYXIN (N=318) |
Exenatide BID (N=316) |
|
|---|---|---|
| HbA1c (%) Baseline (mean) LS Mean change from baseline# LS mean difference vs Exenatide BID# 95% CI |
7.95 -0.73 (0.030 to 0.314) (p=0.0175) |
7.97 -0.90 |
| Patients (%) achieving HbA1c <> | 43.1 | 45.6 |
| Fasting Plasma Glucose (FPG) (mg/dL) Baseline LS Mean change from baselinea |
174.24 -19.79 |
173.88 -24.19 |
| Body weight (kg) Baseline LS Mean change from baselinea |
94.01 -2.74 |
96.09 -3.72 |
ITT population = all randomized patients.
# Using multiple imputation with respect to use the baseline value for missing data at Week 24 in each group.
## Patients with missing HbA1c value at Week 24 were considered non-responders
14% of patients in ADLYXIN and 14% in exenatide had missing HbA1c data at Week 24 in the ITT population
ADLYXIN Prescribing Information
Table 7: Placebo Controlled Study in Patients with Type 2 Diabetes Mellitus in Combination with a Sulfonylurea (24 week results) – ITT Population
| Background therapy | With sulfonylurea +/- metformin* | |
|---|---|---|
| Placebo (N= 286) |
ADLYXIN 20 mcg (N= 573) |
|
| HbA 1c (%) | ||
| Baseline (mean) LS mean change from baseline# Difference from placebo (95% CI) |
8.21 -0.18 |
8.28 -0.77 -0.58 (-0.715, -0.453) (p<> |
| Patients (%) achieving HbA 1c <> | 13 | 33 |
| Fasting Plasma Glucose (FPG) (mg/dL) | ||
| Baseline (mean) LS mean change from baseline# Difference from placebo (95% CI) |
167.47 -10.36 |
174.24 -17.09 -6.73 (-11.946, -1.518) (p=0.0114) |
| Body weight (kg) | ||
| Baseline (mean) LS mean change from baseline# Difference from placebo (95% CI) |
84.34 -0.83 |
82.34 -1.63 -0.80 (-1.244, -0.349) (p=0.0005) |
ITT population = all randomized patients.
# Using multiple imputation with respect to jump to placebo for missing data at Week 24 in the ADLYXIN group.
## Patients with missing HbA1c value at Week 24 were considered non-responders.
* 13% of patients in ADLYXIN and 13% in the placebo had missing HbA1c data at Week 24 in the ITT population
ADLYXIN Prescribing Information
Table 8: Placebo Controlled Study in Patients with Type 2 Diabetes Mellitus in Combination with Pioglitazone (24 week results) – ITT Population
| Background therapy | Pioglitazone +/- metformin* | |
|---|---|---|
| Placebo (N= 161) |
ADLYXIN 20 mcg (N= 323) |
|
| HbA 1c (%) | ||
| Baseline (mean) LS mean change from baseline# Difference from placebo (95% CI) |
8.06 -0.43 |
8.08 -0.91 -0.48 (-0.647, -0.318) (p<> |
| Patients (%) achieving HbA 1c <> | 25 | 49 |
| Fasting Plasma Glucose (FPG) (mg/dL) | ||
| Baseline (mean) LS mean change from baseline# Difference from placebo (95% CI) |
164.49 -14.12 |
164.16 -24.56 -10.45 (-16.580, -4.315) (p=0.0008) |
| Body weight (kg) | ||
| Baseline (mean) LS mean change from baseline# |
96.74 0.26 |
92.93 -0.11 |
ITT population = all randomized patients.
# Using multiple imputation with respect to jump to placebo for missing data at Week 24 in the ADLYXIN group.
## Patients with missing HbA1c value at Week 24 were considered non-responders.
* 9% of patients in ADLYXIN and 12% in the placebo had missing HbA1c data at Week 24 in the ITT population.
ADLYXIN Prescribing Information
Table 9: Placebo Controlled Trials in Patients with Type 2 Diabetes Mellitus in Combination with a Basal Insulin (24 week Treatment Period Results) – ITT Population
| Background therapy | With basal insulin +/-metformin* | With basal insulin +/- sulfonylurea** | |||
|---|---|---|---|---|---|
| Placebo (N= 167) |
ADLYXIN 20 mcg (N= 329) |
Placebo (N= 157) |
ADLYXIN 20 mcg (N= 154) |
||
| HbA1c (%) | |||||
| Baseline (mean) LS mean change from baseline# Difference from placebo (95% CI) |
8.37 -0.34 |
8.42 -0.71 -0.36 (-0.557, -0.170) (p=0.0002) |
8.52 0.07 |
8.54 -0.70 -0.76 (-1.005, -0.516) (p<> |
|
| Patients (%) achieving HbA1c <> | 11 | 25 | 6 | 33 | |
| Fasting Plasma Glucose (FPG) (mg/dL) | |||||
| Baseline (mean) LS mean change from baseline# |
144.94 -13.07 |
146.44 -13.02 |
139.69 2.02 |
138.25 -4.38 |
|
| Body weight (kg) | |||||
| Baseline (mean) LS mean change from baseline# |
88.94 -0.36 |
87.10 -1.55 |
65.60 -0.03 |
65.93 -0.48 |
|
ITT population = all randomized patients.
# Using multiple imputation with respect to jump to placebo for missing data at Week 24 in the ADLYXIN group.
## Patients with missing HbA1c value at Week 24 were considered non-responders.
* 16% of patients in ADLYXIN and 13% in the placebo had missing HbA1c data at Week 24 with basal insulin +/- metformin in the ITT population.
** Conducted in an Asian population. 8 % of patients in ADLYXIN and 6% of patients in placebo had missing HbA1c data at Week 24 with basal insulin +/- sulfonylurea in the ITT population.
ADLYXIN Prescribing Information
Table 10: Placebo Controlled Study in Patients with Type 2 Diabetes Mellitus in Combination with Insulin Glargine (24 week Results) – ITT Population
| Background therapy | With Insulin glargine and metformin +/- Thiazolidinediones* | |
|---|---|---|
| Placebo (N= 223) |
ADLYXIN 20 mcg (N= 223) |
|
| HbA1c (%) | ||
| Baseline (mean) LS mean change from baseline# Difference from placebo (95% CI) |
7.60 -0.42 |
7.56 -0.70 -0.28 (-0.434, -0.123) (p=0.0005) |
| Patients (%) achieving HbA1c <> | 39 | 50 |
| Fasting Plasma Glucose (FPG) (mg/dL) | ||
| Baseline (mean) LS mean change from baseline# |
120.67 6.05 |
117.99 5.74 |
| Body weight (kg) | ||
| Baseline (mean) LS mean change from baseline# Difference from placebo (95% CI) |
86.75 1.09 |
87.31 0.31 -0.78 (-1.388, -0.168) (p=0.0125) |
ITT population = all randomized patients.
# Using multiple imputation with respect to jump to placebo for missing data at Week 24 in the ADLYXIN group.
## Patients with missing HbA1c value at Week 24 were considered non-responders.
* 9% of patients in ADLYXIN and 5% in the placebo had missing HbA1c data at Week 24 in the ITT population.
ADLYXIN Prescribing Information
Table 11: Active Controlled Study in Patients with Type 2 Diabetes Mellitus in Combination with a Basal Insulin with or without Metformin (26 week Treatment Period Results) - ITT population
| ADLYXIN (N=298) |
Insulin Glulisine QD (N=298) |
Insulin Glulisine TID (N=298) |
|
|---|---|---|---|
| HbA1c (%) Baseline LS Mean change from baseline# LS mean difference vs insulin glulisine# 95% CI |
7.77 -0.57 |
7.73 -0.53 -0.04 (-0.161 to 0.080) |
7.79 -0.80 0.23 (0.112 to 0.352) (p=0.0002) |
| Patients (%) achieving HbA1c <> | 38.6 | 36.6 | 47.7 |
| Fasting Plasma Glucose (FPG) (mg/dL) Baseline LS Mean change from baseline# |
118.55 -3.39 |
123.21 -3.68 |
119,80 -1.42 |
| Body weight (kg) Baseline LS Mean change from baseline# LS mean difference vs insulin glulisine# 95% CI |
90.06 -0.64 |
88.45 0.98 |
90.08 1.26 -1.91 (-3.103 to -0.713) (p=0.0018) |
ITT population = all randomized patients. Non-inferiority margin = 0.4%.
# Using multiple imputation with respect to use the baseline value for missing data at Week 26 in each group.
## Patients with missing HbA1c value at Week 26 were considered non-responders.
12% of patients in ADLYXIN, 8% in glulisine QD and 5.0% in glulisine TID had missing HbA1c data at Week 26 in the ITT population.
ADLYXIN Prescribing Information
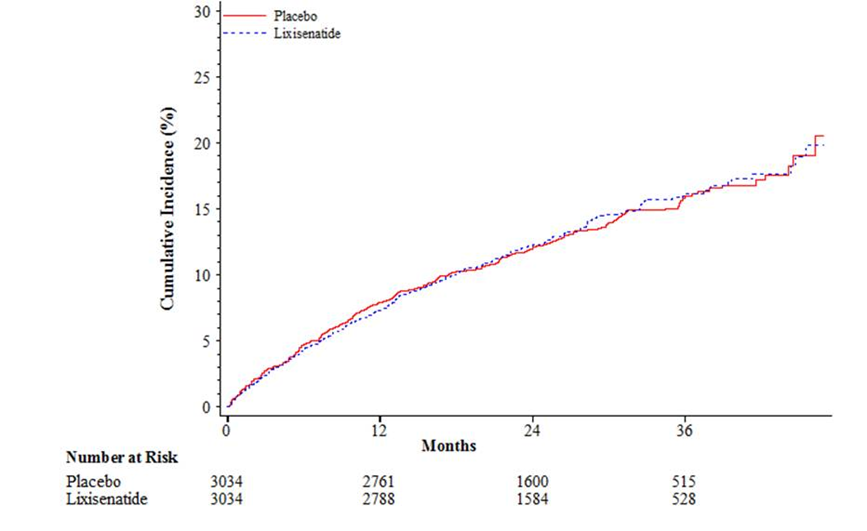
A separate, double-blind, placebo-controlled, non-inferiority trial evaluated cardiovascular (CV) outcomes during treatment with ADLYXIN in patients with type 2 DM after a recent acute coronary syndrome. The primary composite cardiovascular endpoint was the time to the first occurrence of Major Adverse Cardiac Events (MACE+).
Table 12: Analysis of the primary cardiovascular endpoint (time to the first occurrence of the composite of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, or hospitalization for unstable angina) -- ITT population
| Placebo (N=3,034) |
ADLYXIN (N=3,034) |
Hazard ratio (95% CI) |
|
|---|---|---|---|
| Primary composite CV event | 1.02 (0.89, 1.17) |
||
| No. of patients with event (%) | 399 (13.2%) | 406 (13.4%) | |
| Total Person Year | 6328.2 | 6356.8 | |
| Incidence Rate | 6.31 | 6.39 | |
| Component CV event | |||
| Cardiovascular death | 93 (3.1%) | 88 (2.9%) | |
| Non-fatal myocardial infarction | 247 (8.1%) | 255 (8.4%) | |
| Non-fatal stroke | 49 (1.6%) | 54 (1.8%) | |
| Hospitalization for unstable angina | 10 (0.3%) | 9 (0.3%) |
CI: confidence interval, CV: cardiovascular.
Only positively adjudicated events by the Cardiovascular Events Adjudication Committee are included.
ADLYXIN Prescribing Information
Figure 7. Kaplan-Meier cumulative curves of the primary CV endpoint (time to the first occurrence of the composite of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, or hospitalization for unstable angina) - ITT population
ADLYXIN Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: ADLYXIN worked similarly in men and women.
- Race: The majority of patients were White and Asian. ADLYXIN worked slightly better in Asians than in White patients. The number of patients of other races were limited; therefore, differences in response among other races could not be determined
- Age: ADLYXIN worked similarly in patients below and above 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race and age groups?
Figures 8 (for pooled placebo controlled trials) and 9 (active comparator controlled trials) below summarize the results for the primary efficacy endpoint, the change in HbA1c from the start of each trial until the trials were completed for ADLYXIN minus the comparator by sex, age, race, and ethnicity.
Figure 8. Difference (95% Confidence Interval) in Average Change in HbA1c (ADLYXIN minus placebo control)
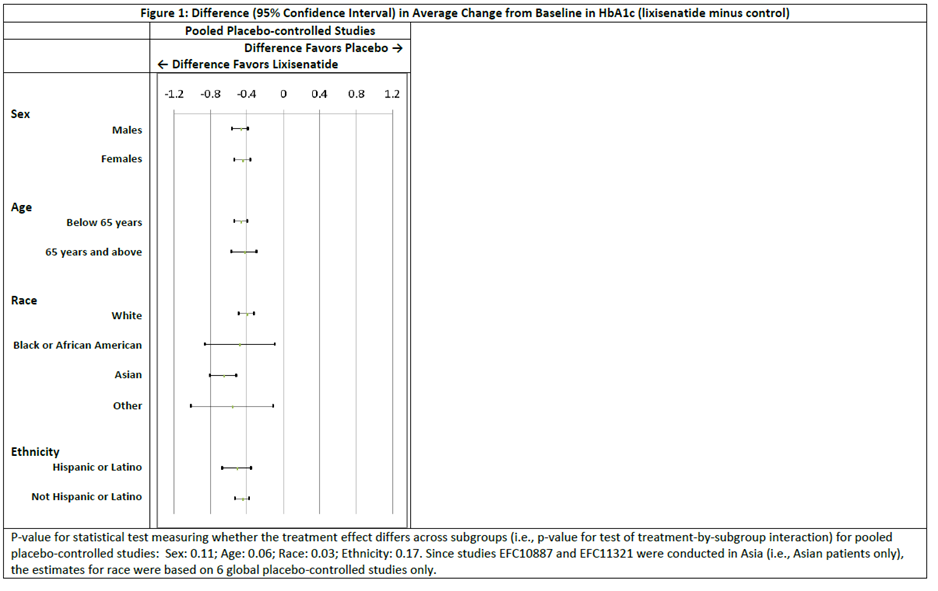
Figure 9. Difference (95% Confidence Interval) in Average Change in HbA1c (ADLYXIN minus active control)
What are the possible side effects?
ADLYXIN may cause serious side effects including severe allergic reactions, inflammation of pancreas and low blood sugar.
The most common side effects are nausea, vomiting, headache, diarrhea and feeling dizzy.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes common adverse reactions for placebo-controlled trial populations. These reflect the Safety population, which includes any patient who received at least one dose of ADLYXIN.
Table 13. Adverse Reactions Reported in ≥5% of ADLYXIN-Treated Patients with Type 2 Diabetes Mellitus and Occurring More Frequently Compared to Placebo
| Adverse reaction | Placebo (N=1639) |
ADLYXIN (N=2869) |
|---|---|---|
| Nausea | 6% | 25% |
| Vomiting | 2% | 10% |
| Headache | 6% | 9% |
| Diarrhea | 6% | 8% |
| Dizziness | 4% | 7% |
ADLYXIN Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The risk of side effects was in similar men and women.
- Race: The majority of patients were White and Asian. The risk of side effects was slightly higher in Asians than in Whites. The number of patients of other races were limited; therefore, differences in response among other races could not be determined.
- Age: The risk of side effects was similar in patients below and above 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race and age groups?
The tables below summarize common adverse events of nausea and vomiting [High level term (HLT): Nausea and vomiting symptoms] by subgroup.
Table 14. HLT: Nausea and Vomiting Symptoms* by Subgroup— Pooled Placebo-Controlled Clinical Trials for the Type 2 DM (safety population)
| Demographic Parameters | ADLYXIN n/N (%) |
Placebo n/N (%) |
|---|---|---|
| Sex | ||
| Men | 298/1362 (22) | 33/811 (4) |
| Women | 504/1507 (33) | 83/828 (10) |
| Age Group | ||
| Below 65 years | 641/2352 (27) | 89/1286 (7%) |
| 65 years and above | 161 (31) | 27 (8) |
| Race | ||
| White | 478/1898 (25) | 78/973 (8) |
| Black or African American | 21/73 (29) | 7/43 (16) |
| Asian | 284/843 (34) | 29/601 (5) |
| Other | 19/55 (35) | 2/22 (9) |
*HLT (High Level Term) includes nausea, vomiting, regurgitation and retching)
Clinical trial data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved ADLYXIN primarily based on evidence from nine clinical trials of 4508 patients with type 2 DM. The trials were conducted in the United States, Canada, Europe, Australia, South America, Africa and Asia.
The FDA also considered data from one separate trial of 6068 patients with type 2 DM who recently suffered heart attack. The trials were conducted in the United States, Canada, Europe, Africa, and Asia.
These two populations will be presented separately
Figures 1-3 below summarize how many patients participated in nine combined clinical trials.
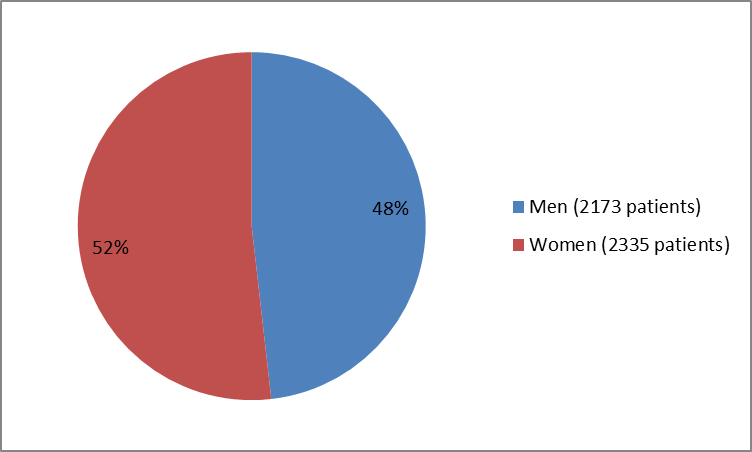
Figure 1. Baseline Demographics by Sex (safety population)
Clinical trial data
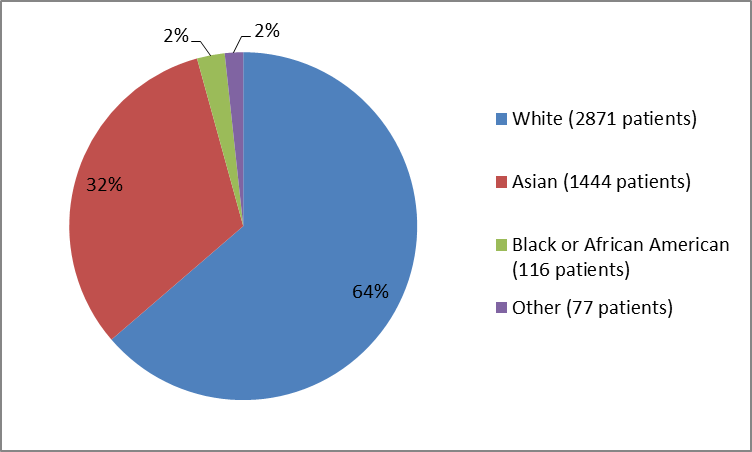
Figure 2. Baseline Demographics by Race (safety population)
Clinical trial data
Table 1. Baseline Demographics by Race (safety population)
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 2871 | 64 |
| Black or African American | 116 | 2 |
| Asian | 1444 | 32 |
| Other | 77 | 2 |
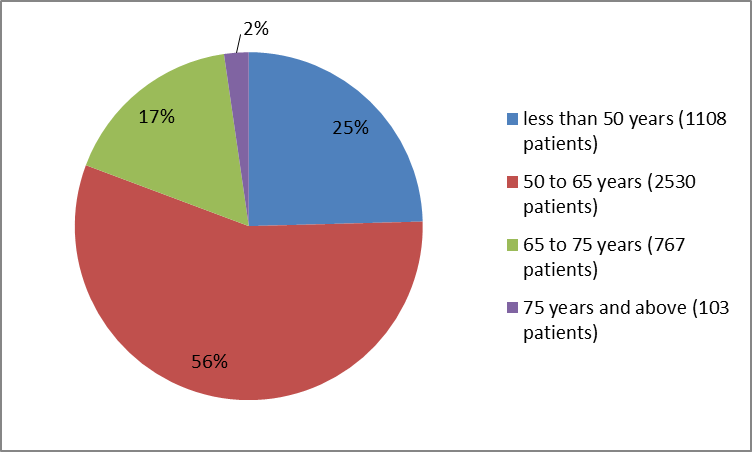
Figure 3. Baseline Demographics by Age (safety population)
Clinical trial data
Figures 4-6 below summarize how many patients by sex, racial and age group participated in the clinical trial used to evaluate patients with type 2 DM who recently suffered heart attack.
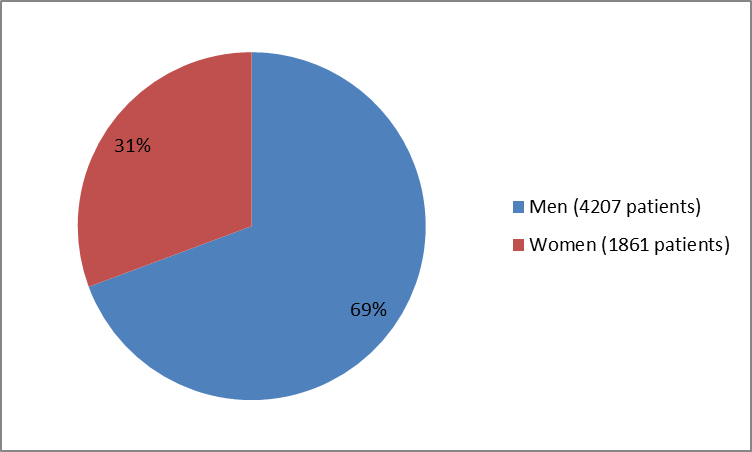
Figure 4. Patients with Type 2 DM and Recent Heart Attack by Sex
Adapted from FDA Statistical review
Figure 5. Patients with Type 2 DM and Recent Heart Attack by Racial Group
Adapted from FDA Statistical review
Table 2. Patients with Type 2 DM and Recent Heart Attack by Racial Group
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 4576 | 75 |
| Black or African American | 221 | 4 |
| Asian | 771 | 13 |
| Other | 500 | 8 |
Adapted from FDA Statistical review
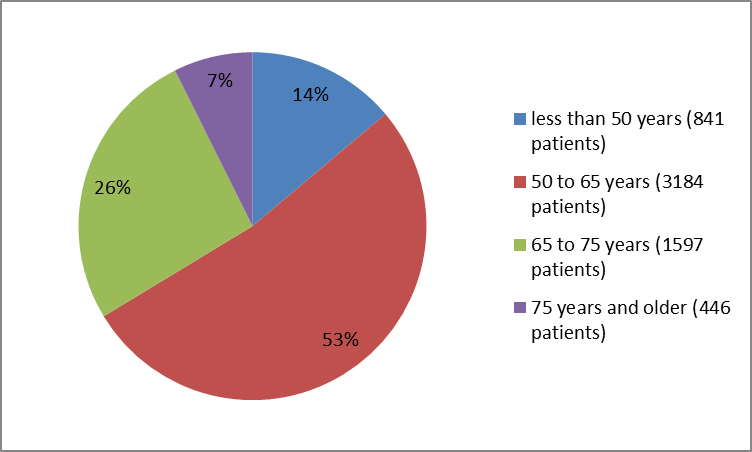
Figure 6. Patients with Type 2 DM and Recent Heart Attack by Age Group
Adapted from FDA Statistical review
Who participated in the trials?
The table below summarizes who participated in 9 pooled placebo-controlled clinical trials of patients with type 2 DM.
Table 15. Baseline Demographics for Pooled Clinical Trials in Patients with Type 2 DM (safety population)
| Demographic Parameter | ADLYXIN N=2869 n (%) |
Placebo N=1639 n (%) |
Total N=4508 n (%) |
|---|---|---|---|
| Sex | |||
| Men | 1362 (47.5) | 811 (49.5) | 2173 (48.2) |
| Women | 1507 (52.5) | 828 (50.5) | 2335 (51.8) |
| Age Group | |||
| <50> | 719 (25.1) | 389 (23.7) | 1108 (24.6) |
| 50 to <65> | 1633 (56.9) | 897 (54.7) | 2530 (56.1) |
| 65 to <75> | 453 (15.8) | 314 (19.2) | 767 (17) |
| 75 years and above | 64 (2.2) | 39 (2.4) | 103 (2.3) |
| Race | |||
| White | 1898 (66.2) | 973 (59.4) | 2871 (63.7) |
| Black or African American | 73 (2.5) | 43 (2.6) | 116 (2.6) |
| Asian | 843 (29.4) | 601 (36.7) | 1444 (32) |
| Other | 55 (1.9) | 22 (1.3) | 77 (1.7) |
| Ethnicity | |||
| Hispanic or Latino | 591 (20.6) | 261 (15.9) | 852 (18.9) |
| Not Hispanic or Latino | 2082 (72.6) | 1184 (72.2) | 3266 (72.4) |
| Unknown/Missing* | 196 (6.8) | 194 (11.8) | 390 (8.7) |
| Region | |||
| United States | 401 (14) | 206 (12.6) | 607 (13.5) |
| Non-US | 2468 (86) | 1433 (87.4) | 3901 (86.5) |
| Canada | 138 (5.6) | 66 (4.6) | 204 (5.2) |
| South and Central America | 528 (21.4) | 253 (17.6) | 781 (20) |
| Europe | 753 (30.5) | 411(28.7) | 1164 (29.8) |
| Africa | 49 (1.9) | 19 (1.3) | 68 (1.7) |
| Asia | 970 (39.3) | 669 (46.7) | 1639 (42) |
| Australia | 30 (1.2) | 15 (1) | 45 (1.2) |
*Some trials did not collect ethnicity information.
Clinical trial data
The table below summarizes demographics from a separate clinical trial of patients with type 2 DM and recent heart attack. Presented is Intention to Treat (ITT) population summary.
Table 16. Baseline Demographics for Trial in Patients with Type 2 DM and Recent Heart Attack (ITT population)
| Demographic Parameter | ADLYXIN N=3034 n (%) |
Placebo N=3034 n (%) |
Total N=6068 n (%) |
|---|---|---|---|
| Sex | |||
| Men | 2111 (69.6) | 2096 (69.1) | 4207 (69.3) |
| Women | 923 (30.4) | 938 (30.9) | 1861 (30.6) |
| Age Group | |||
| <50> | 464 (15.3) | 377 (12.4) | 841 (13.8) |
| 50 to <65> | 1567 (51.6) | 1617 (53.3) | 3184 (52.5) |
| 65 to <75> | 805 (26.5) | 792 (26.1) | 1597 (26.3) |
| 75 years and above | 198 (6.5) | 248 (8.2) | 446 (7.4) |
| Race | |||
| White | 2258 (74.4) | 2318 (76.4) | 4576 (75.4) |
| Black or African American | 118 (3.9) | 103 (3.4) | 221 (3.6) |
| Asian | 404 (13.3) | 367 (12.1) | 771 (12.7) |
| Other | 254 (8.4) | 246 (8.1) | 500 (8.2) |
| Ethnicity | |||
| Hispanic or Latino | 865 (28.5) | 903 (29.8) | 1768 (29.1) |
| Not Hispanic or Latino | 2169 (71.5) | 2131 (70.2) | 4300 (70.7) |
| Region | |||
| North America | 404 (13.3) | 403 (13.3) | 807 (13.3) |
| South and Central America | 972 (32) | 972 (32) | 1944 (32) |
| Western Europe | 354 (11.7) | 377 (12.4) | 731 (12) |
| Eastern Europe | 776 (25.6) | 811 (26.7) | 1587 (26.2) |
| Africa/Near East | 154 (5.1) | 142 (4.7) | 296 (4.9) |
| Asia /Pacific | 374 (12.3) | 329 (10.8) | 703 (11.6) |
Adapted from FDA Statistical review
How were the trials designed?
The benefits and side effects of ADLYXIN for the treatment of adult patients with type 2 DM were evaluated primarily in nine clinical trials. In these trials, patients were randomly assigned to receive either ADLYXIN or placebo injection once daily. Neither the patients nor the health care providers knew which treatment was being given until after the trials were completed. The treatment was given for 24 weeks except in one trial where it was given for 12 weeks. In most trials, patients continued taking their own medications for lowering sugar.
In each trial, the change in HbA1c was measured from the start to finish of the trial and compared between the ADLYXIN and placebo groups in order to evaluate the benefit of ADLYXIN.
There were also two clinical trials where benefit of ADLYXIN was evaluated in comparison to other approved medication for DM type 2. In these two trials, both patients and the health care providers knew which treatment was being given. In one trial, the treatment was given for 24 weeks and in the other one for 26 weeks. In each trial, the change in HbA1c was measured from the start to finish of the trial and compared between the groups.
There was one additional trial where patients with type 2 DM and history of a recent heart attack were treated with either ADLYXIN or a placebo. Neither the patients nor the health care providers knew which treatment was being given until after the trials were completed. This trial compared the rates of cardiovascular (CV) deaths, heart attacks, strokes and hospitalizations due to unstable angina (near heart-attack) between the two groups.
How were the trials designed?
There were eight placebo controlled, randomized, multinational clinical trials where ADLYXIN has been studied as monotherapy, in combination with oral antidiabetic medications, and in combination with basal insulin (with or without oral antidiabetic medications). The primary end-point was reduction in HbA1C measured from the baseline until the end of treatment (12 or 24 weeks).
There were also two open-label, randomized, active controlled trials where ADLYXIN was compared to insulin glulisine (26 week trial) and exenatide (24 week trial). The primary end-point was reduction in HbA1C measured from the baseline until the end of treatment. Both trials were designed as a non-inferiority trials.
Cardiovascular outcome trial was a randomized, double-blind, placebo-controlled, multinational trial that evaluated cardiovascular (CV) outcomes during treatment with ADLYXIN in patients with type 2 diabetes mellitus after a recent Acute Coronary Syndrome. The trial was designed as a non-inferiority trial. Patients were randomized 1:1 to either placebo or ADLYXIN. The primary composite cardiovascular endpoint was the time to the first occurrence of Major Atherosclerotic Cardiovascular Event (MACE), defined as any of the following events positively adjudicated by the Cardiovascular Events Adjudication Committee: cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke, or hospitalization for unstable angina.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.