Drug Trials Snapshot: VABOMERE
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that support the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the VABOMERE Prescribing Information for complete information.
VABOMERE (meropenem and vaborbactam)
(VAY boh meer)
The Medicines Company
Approval date: August 29, 2017
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
VABOMERE is used to treat adults who have a complicated urinary tract infection (abbreviated as cUTI) including infection of the kidneys (pyelonephritis) caused by specific bacteria.
VABOMERE is a combination of two drugs: meropenem a previously-approved drug that fights bacteria and vaborbactam, a new drug that helps meropenem work if the bacteria are resistant to meropenem.
How is this drug used?
VABOMERE is a drug administered by a health care professional directly into the bloodstream through a needle in the vein. This is known as an intravenous, or IV, infusion. It is given every 8 hours for up to 14 days.
What are the benefits of this drug?
After receiving IV treatment with VABOMERE, 98% of patients cured or improved signs and symptoms of cUTI and decreased the number of bacteria in urine in comparison to 94% of patients who received another antibacterial drug piperacillin-tazobactam.
Because cUTI can come back, patients continued with oral antibacterial drugs to complete the treatment for cUTI. The benefit was also evaluated after this treatment was completed (about one week later) and it showed that 76% of patients who were initially treated with VABOMERE and 73% of patients who were initially treated with piperacillin-tazobactam were cured from cUTI.
What are the benefits of this drug (results of trials used to assess efficacy)?
The efficacy of VABOMERE was established based on success at the end of IV treatment (EOIVT) approximately 3-5 days after starting treatment, and was defined as both a clinical outcome of cure or improvement and a microbiologic outcome of eradication (all uropathogens which were at >105/mL at baseline , are to be reduced to 104>
Table 2. Clinical and Microbiological Response Rates in the Trial of cUTI Including Pyelonephritis (m-MITT Population)
| VABOMERE | Piperacillin/ | Difference |
|---|---|---|---|
Clinical cure or | 183/186 (98.4) | 165/175 (94.3) | 4.1% |
Clinical cure AND | 124/162 (76.5) | 112/153 (73.2) | 3.3% |
CI = confidence interval; EOIVT = End of Intravenous Treatment; TOC = Test of Cure
*End of IV Treatment visit includes patients with organisms resistant to piperacillin/tazobactam at baseline
**Test of Cure visit excludes patients with organisms resistant to piperacillin/tazobactam at baseline
VABOMERE Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: VABOMERE worked similarly in men and women.
- Race: The majority of patients were White. The number of patients in other races was limited. Differences in how well the drug worked among races could not be determined.
- Age: VABOMERE worked similarly in patients below and above 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race and age groups?
Subgroup analysis at the TOC (test of cure) time point for Clinical cure and Microbiological Eradication endpoints are presented below.
Table 3. Summary of Clinical Cure at the TOC visit in Demographic Subgroups of the m-MIT Population
| Subgroup | VABOMERE | Piperacillin /Tazobactam | Difference | 95% confidence interval |
|---|---|---|---|---|
| Sex | ||||
| Men | 58/67 (86.6%) | 55/62 (88.7%) | -2.1% | -14.0% to 9.9% |
| Women | 116/125 (92.8%) | 102/120 (85.0%) | 7.8% | 0.0% to 16.1% |
| Race | ||||
| White | 162/178 (91.0%) | 147/169 (87.0%) | 4.0% | -2.6% to 10.9% |
| Black | 3/3 (100%) | 2/2 (100%) | - | |
| Asian | 4/4 (100%) | 2/3 (66.7%) | - | |
| Other | 5/7 (71.4%) | 6/8 (75.0%) | - | |
| Age(years) | ||||
| 122/130 (93.8%) | 96/105 (91.4%) | 2.4% | -4.4% to 10.0% | |
| 65-74 | 30/35 (85.7%) | 30/39 (76.9%) | 8.8% | -9.7% to 26.6% |
| ≥75 | 22/27 (81.5%) | 31/38 (81.6%) | -0.1% | -20.9% to 18.6% |
Table 4. Summary of Microbiological Eradication at the TOC visit in Demographic Subgroups of the m-MITT Population
| Subgroup | VABOMERE | Piperacillin/Tazobactam | Difference | 95% confidence interval | |
|---|---|---|---|---|---|
| Sex | |||||
| Men | 44/67 (65.7%) | 40/62 (64.5%) | 1.2% | -15.1% to 17.5% | |
| Women | 88/125 (70.4%) | 73/120 (60.8%) | 9.6% | -2.3% to 21.3% | |
| Race | |||||
| White | 127/178 (71.3%) | 105/169 (62.1%) | 9.2% | -0.7% to 19% | |
| Black | 2/3 (66.7%) | 2/2 (100%) | - | ||
| Asian | 1/4 (25.0%) | 1/3 (33.3%) | - | ||
| Other | 2/7 (28.6%) | 5/8 (62.5%) | - | ||
| Age(years) | |||||
| 95/130 (73.1%) | 75/105 (71.4%) | 1.6% | -9.7% to 13.3% | ||
| 65-74 | 20/35 (57.1%) | 19/39 (48.7%) | 8.4% | -14.1% to 30.1% | |
| ≥75 | 17/27 (63.0%) | 19/38 (50.0%) | 13.0% | -11.5% to 35.5% | |
FDA Statistical review
What are the possible side effects?
VABOMERE can cause serious and life threatening allergic reactions. Other serious side effects include seizures and severe diarrhea caused by C. difficile.
Common side effects that were associated with the use of VABOMERE include headache, infusion site reactions, and diarrhea.
What are the possible side effects (results of trials used to assess safety)?
Table 5 summarizes the most common adverse events observed in the trial.
Table 5. Adverse Reactions Occurring in 1% or Greater of Patients Receiving VABOMERE in the Clinical Trial in cUTI
Selected Adverse Reactions | VABOMERE | Piperacillin/Tazobactama |
|---|---|---|
Headache | 8.8 | 4.4 |
Phlebitis/Infusion site reactionsb | 4.4 | 0.7 |
Diarrhea | 3.3 | 4.4 |
Hypersensitivityc | 1.8 | 1.8 |
Nausea | 1.8 | 1.5 |
Alanine aminotransferase increased | 1.8 | 0.4 |
Aspartate aminotransferase increased | 1.5 | 0.7 |
Pyrexia | 1.5 | 0.7 |
Hypokalemia | 1.1 | 1.5 |
a Piperacillin/tazobactam 4.5 g (piperacillin 4g/tazobactam 0.5g) IV infused over 30 minutes every 8 hours.
b Infusion site reactions include infusion/injection site phlebitis, infusion site thrombosis, and infusion site erythema.
c Hypersensitivity includes hypersensitivity, drug hypersensitivity, anaphylactic reaction, rash urticaria, and bronchospasm.
VABOMERE Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar between men and women.
- Race: The majority of patients were White. The number of patients in other races was limited. Differences in side effects among races could not be determined.
- Age: The occurrence of side effects was similar in patients below and above 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race and age groups?
The tables below summarizes the incidence of treatment-emergent adverse events (adverse events that occurred while patient was receiving study drug treatment) and serious adverse events in the clinical trial by subgroup.
Table 6. Summary of Incidence of Treatment-Emergent Adverse Events by Subgroup (Safety Population)
| Subgroup | VABOMERE N=272 | Piperacillin/ Tazobactam N=273 | Relative Risk (95% CI) | |||
|---|---|---|---|---|---|---|
| n(%) | Total,N | n(%) | Total,N | |||
| Overall | 106 (39.0) | 272 | 97 (35.5) | 273 | 1.10 (0.88, 1.36) | |
| SEX | ||||||
| Men | 31 (34.1) | 91 | 24 (25.8) | 93 | 1.32 (0.84, 2.07) | |
| Women | 75 (41.4) | 181 | 73 (40.6) | 180 | 1.02 (0.80, 1.31) | |
| RACE | ||||||
| White | 93 (36.6) | 254 | 85 (33.7) | 252 | 1.09 (0.86, 1.38) | |
| Black or African American | 1 (33.3) | 3 | 3 (75.0) | 4 | 0.44 (0.08, 2.43) | |
| Asian | 3 (60.0) | 5 | 2 (40.0) | 5 | 1.50 (0.41, 5.45) | |
| Other | 9 (90.0) | 10 | 7 (58.3) | 12 | 1.54 (0.92, 2.60) | |
| AGE (years) | ||||||
| 74 (40.0) | 185 | 62 (36.5) | 170 | 1.10 (0.84, 1.43) | ||
| >= 65 | 32 (36.8) | 87 | 35 (34.0) | 103 | 1.08 (0.74, 1.59) | |
Table 7. Summary of Incidence of Serious Adverse Events by Subgroup (Safety Population)
| Subgroup | VABOMERE N=272 | Piperacillin/ Tazobactam N=273 | Relative Risk (95% CI) | ||
|---|---|---|---|---|---|
| n(%) | Total,N | n(%) | Total,N | ||
| OVERALL | 11 (4.0) | 272 | 12 (4.4) | 273 | 0.92 (0.41, 2.05) |
| SEX | |||||
| Men | 8 (8.8) | 91 | 2 (2.2) | 93 | 4.09 (0.89, 18.73) |
| Women | 3 (1.7) | 181 | 10 (5.6) | 180 | 0.30 (0.08, 1.07) |
| RACE | |||||
| White | 10 (3.9) | 254 | 10 (4.0) | 252 | 0.99 (0.42, 2.34) |
| Black or African American | 0 (0.0) | 3 | 1 (25.0) | 4 | -- |
| Asian | 0 (0.0) | 5 | 0 (0.0) | 5 | -- |
| Other | 1 (10.0) | 10 | 1 (8.3) | 12 | 1.20 (0.09, 16.84) |
| AGE (years) | |||||
| 4 (2.2) | 185 | 6 (3.5) | 170 | 0.61 (0.18, 2.13) | |
| >= 65 | 7 (8.0) | 87 | 6 (5.8) | 103 | 1.38 (0.48, 3.96) |
Clinical trial data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
FDA approved VABOMERE based on the trial (NCT02166476) of 545 patients with cUTI. The trial included patients from the Europe, North America, Asia and South America.
Figure 1 summarizes how many men and women were in the clinical trial.
Figure 1. Baseline Demographics by Sex
FDA Clinical review
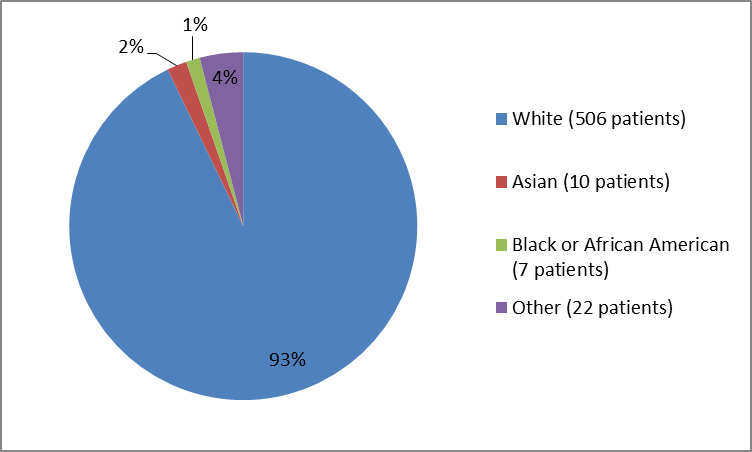
Figure 2 and Table 1 summarize the percentage of patients by race in the clinical trial.
Figure 2. Baseline Demographics by Race
FDA Clinical review
Table 1. Baseline Demographics by Race
Race | Number of Patients | Percentage (%) |
|---|---|---|
White | 506 | 93 |
Black / African American | 7 | 1 |
Asian | 10 | 2 |
Other | 22 | 4 |
FDA Clinical review
Figure 3 summarizes by age how many patients were in the clinical trial
Figure 3. Baseline Demographics by Age
Who participated in the trials?
The table below summarizes baseline demographic information for the safety population defined as all patients who received at least one dose of the treatment.
Table 8. Demographic and Baseline Characteristics (Safety Population)
| DemographicParameters | VABOMERE N=272 n (%) | Piperacillin- Tazobactam N=273 n (%) | TOTAL N=545 n (%) |
|---|---|---|---|
| Sex | |||
| Women | 181 (66.5) | 180 (66) | 361 (66) |
| Men | 91 (33.5) | 93 (34) | 184 (34) |
| Race | |||
| White | 254 (93) | 252 (92) | 506 (93) |
| Asian | 5 (2) | 5 (2) | 10 (2) |
| Black or African American | 3 (1) | 4(1) | 7 (1) |
| Other | 10 (4) | 12 (4) | 22 (4) |
| Age | |||
| Median (min, max) | 58 (18,92) | 57 (18,94) | 58 (18,94) |
| Age Group | |||
| 185 ( 68) | 170 (62) | 355 (65) | |
| 65- | 48 (18) | 57 (21) | 105 (19) |
| >= 75 years | 39 ( 14) | 46 (17) | 85 (16) |
| Ethnicity | |||
| Hispanic or Latino | 24 (9) | 19 (7) | 43 (8) |
| Not Hispanic or Latino | 248 (91) | 254 (93) | 502 (92) |
| Region | |||
| Europe | 244 (90) | 243 (89) | 487 (89) |
| North America | 8 (3) | 9 (3) | 17 (3) |
| Asia Pacific | 4 (1) | 5 (2) | 9 (2) |
| Rest of World | 16 (6) | 16 (6) | 32 (6) |
FDA Clinical review
How were the trials designed?
In the clinical trial, half of the patients were chosen at random to receive VABOMERE, and the other half was given another antibacterial drug called piperacillin-tazobactam. Both treatments were given intravenously every 8 hours for up to 14 days and neither the patients nor the health care professionals knew which drugs were given until after the study was complete. After 5 days of IV treatment with VABOMERE or piperacillin-tazobactam, patients could be switched to other oral antibacterial drugs to complete the treatment for cUTI.
The benefit of VABOMERE was measured by the proportion of patients who achieved cure or improvement in their symptoms related to cUTI and a negative urine culture test and compared it to piperacillin-tazobactam.
How were the trials designed?
There was one randomized, active-controlled, double blind, double dummy, multi center trial comparing VABOMERE to piperacillin tazobactam in the treatment of adults with cUTI. Medications were given intravenously every 8 hours for up to 14 days. A switch to levofloxacin or other approved oral treatment was allowed after a minimum of 15 doses of IV therapy.
The efficacy was based on non-inferiority assessment of the primary-endpoint of success at the end of IV treatment (EOIVT) which required both a clinical outcome of cure or improvement and a microbiologic outcome of eradication (all baseline uropathogens at >105/mL are to be reduced to 104>
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.