Drug Trials Snapshot: Savaysa (edoxaban) for Prevention of Stroke in Atrial Fibrillation
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the SAVAYSA Package Insert for complete information.
DRUG TRIALS SUMMARY:
SAVAYSA (edoxaban)
For Prevention of Stroke in Atrial Fibrillation
Sa-VAYE-sah
Daiichi Sankyo, Inc.
Approval date: January 8, 2015
What is the drug for?
People with atrial fibrillation (a type of irregular heart rhythm) are at an increased risk of forming a blood clot in the heart that can travel to either their brain, leading to a stroke, or can travel to other parts of their body, causing a serious blockage in a blood vessel. SAVAYSA is a drug known as a “blood thinner” that lowers the chance of having a stroke or other blood vessel blockage by helping to prevent clots from forming in the heart.
How do I use this drug?
SAVAYSA is a pill taken once a day.
What are the benefits of this drug?
The clinical trial that supported FDA’s approval of SAVAYSA compared two different doses of SAVAYSA to warfarin, a drug that is used to decrease the chance of a stroke or blood vessel obstruction in patients with atrial fibrillation. Overall, the higher dose of SAVAYSA was similarly effective to warfarin, and with SAVAYSA there were also fewer strokes caused by bleeding into the brain. The lower dose of SAVAYSA was less effective than warfarin.
Importantly, a patient’s kidney function should be checked before starting SAVAYSA. People whose kidneys work really well should not receive SAVAYSA because it does not work well to prevent stroke in such patients.
What are the benefits of this drug (results of trials used to assess efficacy)?
In the trial, both doses of SAVAYSA were shown to be non-inferior to warfarin in reducing the risk of stroke or systemic embolic event in patients with atrial fibrillation, but the higher dose was better than the lower dose and warfarin. Table 2 summarizes these overall results, showing that the pre-specified non-inferiority criteria (upper bound of the 95% confidence interval [CI] < 1.38) were met for the primary efficacy endpoint in the mITT population in both edoxaban groups. However, only the higher dose of SAVAYSA was approved because of its greater efficacy in preventing stroke and SEE compared to the lower dose. It is also notable that for ischemic stroke, the lower dose was markedly inferior to warfarin, although it had a clear advantage for hemorrhagic stroke.
Table 2. Strokes and Systemic Embolic Events in the Clinical Trial (mITT, On-treatment)
|
|
SAVAYSA |
SAVAYSA |
Warfarin (N=7012) n (%/yr) b |
SAVAYSA 30 mg vs. warfarin |
SAVAYSA 60 mg vs. warfarin HR (CI) c |
|---|---|---|---|---|---|
|
First Stroke or SEE |
253 (1.6) |
182 (1.2) |
232 (1.5) |
1.07 (0.87, 1.31) |
0.79 (0.63, 0.99) |
|
Ischemic Stroke |
225 (1.4) |
135 (0.9) |
144 (0.9) |
1.54 (1.25, 1.90) |
0.94 (0.75, 1.19) |
|
Hemorrhagic |
18 (0.1) |
39 (0.3) |
75 (0.5) |
0.24 (0.14, 0.39) |
0.52 (0.36, 0.77) |
|
Systemic |
10 (<> |
8 (<> |
13 (<> |
0.75 (0.33, 1.72) |
0.62 (0.26, 1.50) |
Abbreviations: HR = Hazard Ratio versus warfarin, CI = Confidence Interval, n = number of events, mITT = Modified Intent-to-Treat, N=number of patients in mITT
population, SEE = Systemic Embolic Event, yr = year
a Includes patients dose-reduced to 15 mg for the 30 mg treatment group and 30 mg for the 60 mg treatment group
b The event rate (%/yr) is calculated as number of events/subject-year exposure.
c 97.5% CI for primary endpoint of First Stroke or SEE. 95% CI for Ischemic Stroke, Hemorrhagic Stroke or Systemic Embolism
Source: SAVAYSA Package Insert
SAVAYSA worked better in patients with mildly impaired kidney function than in patients with normal kidney function. The study showed that the higher dose (60 mg) was superior to the lower dose (30 mg), an advantage related to the higher SAVAYSA blood levels attained. Because SAVAYSA is eliminated from the body by the kidneys, people with normal kidney function excrete it more efficiently and end up with blood levels of SAVAYSA that are approximately 30% lower than patients with mildly abnormal kidney function.
As a result, patients with normal kidney function had ischemic stroke rates considerably higher than those who were randomized to warfarin, in contrast to patients with mildly impaired kidney function, who had a considerably lower stroke rate than those who were randomized to warfarin. Blood levels in patients with creatinine clearance more than 95 milliliters per minute are not adequate to optimally prevent stroke and systemic embolism. SAVAYSA is not indicated in such patients. Patients with creatinine clearance more than 95 milliliters per minute should use another anticoagulant drug.
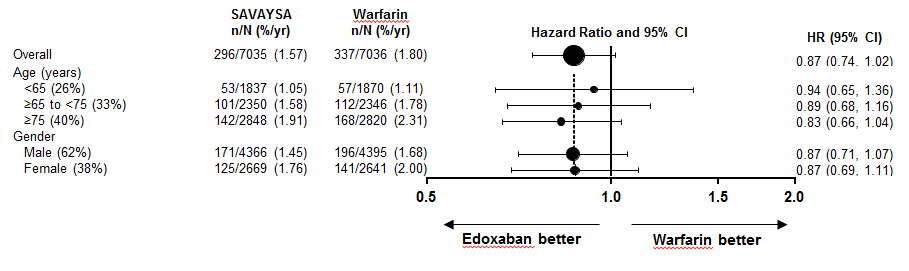
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
Subgroup analyses were conducted for sex, race, and age subgroups.
- Sex: SAVAYSA was similarly effective in men and women.
- Race: SAVAYSA was similarly effective in Whites and Asians. Because the number of non-White, non-Asian patients in the trial was limited, it was not possible to determine whether there were clinically meaningful differences.
- Age: SAVAYSA was similarly effective in patients above and below age 75.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age?
Figure 3. Primary Efficacy Endpoint by Subgroups for Clinical Trial (Intent to Treat Population)
Source: SAVAYSA Package Insert
Abbreviations: yr =year, CI = confidence interval
What are the possible side effects?
The most common side effect of SAVAYSA during clinical trials was bleeding, which can be serious. This is because SAVAYSA is a medicine that interferes with the process of blood clotting in the body, making bleeding more likely.
What are the possible side effects (results of trials used to assess safety)?
The most common non-bleeding adverse reactions for SAVAYSA 60 mg versus warfarin were rash (4.2% vs. 4.1%, respectively), and abnormal liver function tests (4.8% vs. 4.6%, respectively).
The primary safety endpoint of the study was Major Bleeding that occurred during administration of SAVAYSA and/or for up to 3 days following its discontinuation. SAVAYSA was better than warfarin for this primary safety endpoint. Table 3 summarizes the primary safety analysis results in the population for whom the drug is indicated (patients with CrCL ≤ 95 mL/minute). SAVAYSA also had lower event rates than warfarin for clinically relevant non-Major (CRNM) bleeding, except for major gastrointestinal tract bleeding, where the rate was higher than with warfarin.
Table 4. Primary Major Bleeding Results—On treatment, Safety Set
|
Event a |
SAVAYSA 60 mgb |
Warfarin |
SAVAYSA 60 mg vs. Warfarin |
|---|---|---|---|
|
Major Bleedingc |
357 (3.1) |
431 (3.7) |
0.84 (0.73, 0.97) |
|
Intracranial |
53 (0.5) |
122 (1.0) |
0.44 (0.32, 0.61) |
|
Hemorrhagic |
33 (0.3) |
69 (0.6) |
0.49 (0.32, 0.74) |
|
Other ICH |
20 (0.2) |
55 (0.5) |
0.37 (0.22, 0.62) |
|
Gastrointestinal |
205 (1.8) |
150 (1.3) |
1.40 (1.13, 1.73) |
|
Fatal Bleeding |
21 (0.2) |
42 (0.4) |
0.51 (0.30, 0.86) |
|
ICH |
19 (0.2) |
36 (0.3) |
0.54 (0.31, 0.94) |
|
Non- intracranial |
2 (<> |
6 (<> |
---- |
|
CRNM Bleedinge |
982 (9.4) |
1132 (10.9) |
0.87 (0.80, 0.95) |
Abbreviations: HR = Hazard Ratio versus Warfarin, CI = Confidence Interval, n = number of patients with events, N = number of patients in Safety population, CRNM = Clinically Relevant Non-Major.
* During or within 2 days of stopping study treatment
a A subject can be included in multiple sub-categories if he/she had an event for those categories.
b Includes all patients with CrCL ≤ 95 mL/min randomized to receive 60 mg once daily, including those who were dose-reduced to 30 mg once daily because of prespecified baseline conditions
c Defined as a clinically overt bleeding that met one of the following criteria: fatal bleeding; symptomatic bleeding in a critical site such as retroperitoneal, intracranial, intraocular, intraspinal, intra-articular, pericardial, or intramuscular with compartment syndrome; a clinically overt bleeding event that caused a fall in hemoglobin of at least 2.0 g/dL (or a fall in hematocrit of at least 6.0% in the absence of hemoglobin data), when adjusted for transfusions (1 unit of transfusion = 1.0 g/dL drop in hemoglobin).
d ICH includes primary hemorrhagic stroke, subarachnoid hemorrhage, epidural/subdural hemorrhage, and ischemic stroke with major hemorrhagic conversion.
e Defined as an overt bleeding event that did not meet the criteria for Major Bleed, but required medical attention, including bleeding that may have resulted in diagnostic or therapeutic measures.
Source: SAVAYSA Package Insert
Despite a better bleeding profile, the rate of anemia-related adverse events was greater with high-dose SAVAYSA than with warfarin (9.6% vs. 6.8%).
Interstitial Lung Disease (ILD) was reported as a serious adverse event for SAVAYSA 60 mg and warfarin in 15 (0.2%) and 7 (0.1%) patients, respectively. Many of the cases in both treatment groups were confounded by the use of amiodarone, which has been associated with ILD, or by infectious pneumonia. In the overall study period, there were 5 and 0 fatal ILD cases in the SAVAYSA 60 mg and warfarin groups, respectively.
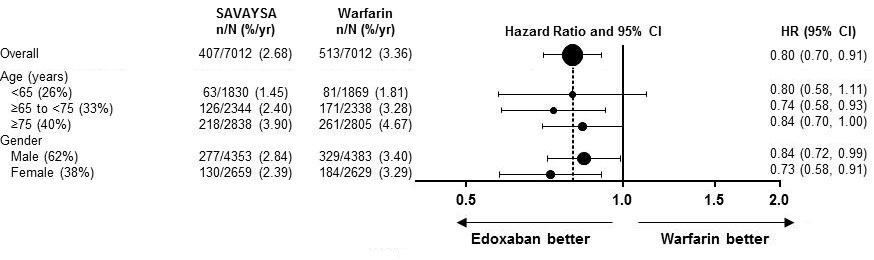
Were there any differences in side effects among sex, race and age?
Subgroup analyses were conducted for sex, race and age.
- Sex: The risk of major bleeding was similar among men and women.
- Race: The risk of major bleeding was similar between Whites and Asians. Because the number of non-White, non-Asian patients in the trial was limited, it was not possible to determine whether there were clinically meaningful differences in bleeding.
- Age: Subgroup analysis for major bleeding was conducted for ages below 65, ages 65 to 74, and 75 years and above. The risk of major bleeding was similar across these three age groups.
Were there any differences in major bleeding among sex, race, and age subgroups?
The results of major bleeding for the high-dose SAVAYSA and warfarin are summarized in Figure 4.
Figure 4. Major Bleeding by subgroup in the clinical trial
Source: SAVAYSA Package Insert
Abbreviations: yr =year, CI = confidence interval
WHO WAS IN THE STUDIES?
Who participated in the clinical trials?
The FDA approved SAVAYSA based on evidence from a clinical trial of 21,026 patients with atrial fibrillation. The study was conducted at 1,393 sites in 46 countries in North America, South America, Europe, Asia Pacific, and South Africa.
Figure 1 summarizes how many men and women were enrolled in the clinical trial.
Figure 1. Baseline Demographics by Sex
Source: Extracted from FDA Clinical Review, Table 2
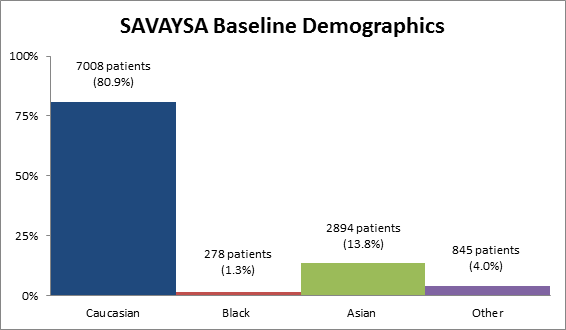
Figure 2 summarizes the percentage of patients by race enrolled in the clinical trial.
Figure 2. Baseline Demographics by Race
Source: Extracted from FDA Clinical Review, Table 2
Table 1. Demographics of Efficacy Trials by Race
Race |
Number of Patients |
Percentage |
|---|---|---|
|
Caucasian |
17,008 |
80.9% |
|
Black |
278 |
1.3% |
|
Asian |
2894 |
13.8% |
|
Other |
845 |
4.0% |
Source: Extracted from FDA Clinical Review, Table 2
Who participated in the study?
SAVAYSA was evaluated in one clinical trial to look at the reduction in the risk of stroke and systemic embolization in patients with nonvalvular atrial fibrillation (referred to here as atrial fibrillation). The trial population 1included 21,026 patients with atrial fibrillation, stratified by CHADS2 risk score, a measure indicating the likelihood of a stroke,2of whom 7,002 were randomized to the low-dose SAVAYSA (30 mg) treatment arm, 7,012 were randomized to the high-dose SAVAYSA (60 mg) arm, and 7,012 were randomized to the warfarin arm.3
The trial was conducted at 1,393 sites in 46 countries in North America, South America, Europe, Asia Pacific, and South Africa. The demographic characteristics are summarized in Table 4.
Table 4. Baseline Demographic Characteristics
| SAVAYSA 30 mg (15mg DA*) (N=7002) |
SAVAYSA 60 mg (30mg DA*) (N=7012) |
Warfarin (N=7012) |
|
|---|---|---|---|
| Age (years), n | 7002 | 7012 | 7012 |
|
Mean (SD) |
70.6 (9.3) | 70.6 (9.5) | 70.5 (9.4) |
|
Median |
72.0 | 72.0 | 72.0 |
|
Minimum, Maximum |
27. 95 | 25. 96 | 27. 95 |
|
>= 65 years, n (%) |
5218 (74.5) | 5182 (73.9) | 5143 (73.3) |
|
>= 75 years, n (%) |
2789 (39.8) | 2838 (40.5) | 2805 (40.0) |
|
>= 80 years, n (%) |
1197 (17.1) | 1177 (16.8) | 1195 (17.0) |
| Sex | |||
|
Male, n (%) |
4284 (61.2) | 4353 (62.1) | 4383 (62.5) |
|
Female, n (%) |
2718 (38.8) | 2659 (37.9) | 2629 (37.5) |
| Race | |||
|
Caucasian, n (%) |
5650 (80.7) | 5679 (81.0) | 5679 (81.0) |
|
Black, n (%) |
94 (1.3) | 96 (1.4) | 88 (1.3) |
|
Asian, n (%) |
975 (13.9) | 956 (13.6) | 963 (13.7) |
|
Other, n (%) |
282 (4.0) | 281 (4.0) | 282 (4.0) |
| Region | |||
|
North America, n (%) |
1550 (22.1) | 1559 (22.2) | 1556 (22.2) |
|
USA, n (%) |
1308 (18.7) | 1288 (18.4) | 1297 (18.5) |
|
Latin America, n (%) |
882 (12.6) | 884 (12.6) | 885 (12.6) |
|
Western Europe, n (%) |
1075 (15.4) | 1075 (15.3) | 1070 (15.3) |
|
Eastern Europe, n (%) |
2369 (33.8) | 2374 (33.9) | 2378 (33.9) |
|
Asia/Pacific and South Africa, n (%) |
789 (11.3) | 784 (11.2) | 786 (11.2) |
| Japan, n (%) | 337 (4.8) | 336 (4.8) | 337 (4.8) |
2The CHADS2 risk score represents the sum of the following risk factors for stroke: congestive heart failure, hypertension, age >75 years, diabetes mellitus (1 point each), and previous stroke or transient ischemic attack (2 points). Patients were stratified at randomization into: Stratum 1 – CHADS2 risk score 2 and 3, and Stratum 2 – CHADS2 risk score 4, 5, and 6.
3Patients with one or more factors at screening requiring dose adjustment (body weight 60 kg, creatinine clearance 30-50 mL/min, concomitant treatment with P-glycoprotein (gp) inhibitors) received half of the regular edoxaban dosage regimen (i.e., in the high-dose edoxaban group, patients on full dose received edoxaban 60 mg once daily, and patients requiring dose adjustment received 30 mg once daily; in the low-dose edoxaban group, patients on full dose received edoxaban 30 mg once daily, and patients requiring dose adjustment received 15 mg once daily).
Extracted from FDA Clinical Review, Table 2
How was the study designed?
The trial that compared SAVAYSA to warfarin enrolled 21,026 patients diagnosed with atrial fibrillation. One-third of patients was randomly assigned to a low dose of SAVAYSA, one-third was assigned a high dose of SAVAYSA, and another third was assigned to warfarin. Neither the patients nor the health care professionals knew which treatment each patient was receiving until after the trial was complete. Patients were evaluated for approximately 2.8 years.
The efficacy of SAVAYSA was evaluated in one randomized, double-blind, controlled Phase 3 clinical trial comparing the effect of SAVAYSA 30 mg, SAVAYSA 60 mg, and warfarin on time to first stroke (of any kind) or systemic embolic event (SEE). Strokes and SEE were counted while patients were on treatment and for the first 3 days after drug discontinuation. The primary statistical analyses were the following two comparisons:
- SAVAYSA high-dose compared to warfarin, and
- SAVAYSA low-dose compared to warfarin
Doses in the SAVAYSA arms were reduced by half if patients were expected to have higher blood levels for one of the following reasons: low body weight, poor excretion of the drug because of impaired kidney function, or use of other drugs that might raise SAVAYSA blood levels.
Patients with atrial fibrillation must be treated with an anticoagulant to avoid serious consequences (such as a stroke). Because of this, the trial could not compare SAVAYSA with a placebo. Therefore it compared SAVAYSA to warfarin, a known effective drug, attempting to show SAVAYSA is not worse than warfarin. This is called a “non-inferiority study.” In this case the degree of inferiority to be ruled out statistically was a 38% worse effect on stroke plus systemic embolic events compared to warfarin, a degree of inferiority that would represent loss of about half the effect of warfarin.
SAVAYSA was evaluated for safety in the same population used to assess efficacy.
What are the results of the efficacy study?
In the overall study, high-dose SAVAYSA was about as effective as warfarin at preventing strokes and other blood vessel obstruction, but was less effective than warfarin in people with better kidney function. A kidney function test should be given to help health care providers determine whether they should use edoxaban or another anticoagulant.
What are the results of the trials used to assess safety?
Patients who took either dose of SAVAYSA had significantly fewer problems with bleeding than those who took warfarin.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PACKAGE INSERT
MEDICAL REVIEW