Drug Trials Snapshot: PRALUENT
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the PRALUENT Prescribing Information for complete information.
PRALUENT (alirocumab)
(PRAHL-u-ent)
Sanofi
Approval date: July 24, 2015
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
PRALUENT is a drug for the treatment of high LDL cholesterol, which is sometimes referred to as “bad cholesterol”.
PRALUENT is to be used in the following two groups of patients: 1) patients with an inherited condition called heterozygous familial hypercholesterolemia (HeFH) or 2) patients who have had complications of having too much cholesterol, such as heart attacks and strokes. It is approved to be used in addition to diet and the highest dose of a statin (another drug commonly used to lower cholesterol) that a patient can tolerate, and only when LDL cholesterol needs to be lowered further.
How is this drug used?
PRALUENT is an injection that is given under the skin in the thigh, abdomen, or upper arm once every two weeks.
What are the benefits of this drug?
PRALUENT lowers LDL cholesterol.
What are the benefits of this drug?
The efficacy of PRALUENT was investigated in five double-blind, placebo-controlled trials that enrolled 3499 patients. Table 2 below summarizes the results for the primary efficacy endpoint, which was the mean percent change in LDL-C from baseline to week 24 for PRALUENT compared with placebo, for each trial.
Table 2. Percent Change in LDL C at Week 24 by Trial
| Baseline (mg/dL) |
LS Mean: % Change |
Difference: PRALUENT minus placebo (95% CI) |
|
|---|---|---|---|
| Trial 1 | |||
| PRALUENT 150mg (N=1553) | 123 | -58% | |
| Placebo (N=788) | 122 | 1% | -58% (-61, -56) |
| Trial 2 | |||
| PRALUENT 75mg/150* mg (N=209) | 100 | -44% | |
| Placebo (N=107) | 105 | -2% | -43% (-50, -35) |
| Trial 3 (HeFH only) | |||
| PRALUENT 75mg/150mg* (N=323) | 145 | -47% | |
| Placebo (N=163) | 144 | 9% | -56% (-62, -51) |
| Trial 4 (HeFH only) | |||
| PRALUENT 75mg/150mg* (N=167) | 135 | -47% | |
| Placebo (N=82) | 134 | 3% | -50% (-57, -43) |
| Trial 5 (HeFH only) | |||
| PRALUENT 150mg (N=72) | 196 | -43% | |
| Placebo (N=35) | 201 | -7% | -36% (-49, -24) |
*Dosing reflects possible up-titration from 75 mg to 150 mg at week 12.
Source: FDA Statistical Review
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
Subgroup analyses were conducted for sex, race, and age.
- Sex: PRALUENT worked similarly in men and women.
- Race: The majority of patients in the trials were white. Differences in response to PRALUENT among races could not be determined.
- Age: PRALUENT worked similarly in all age groups studied.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
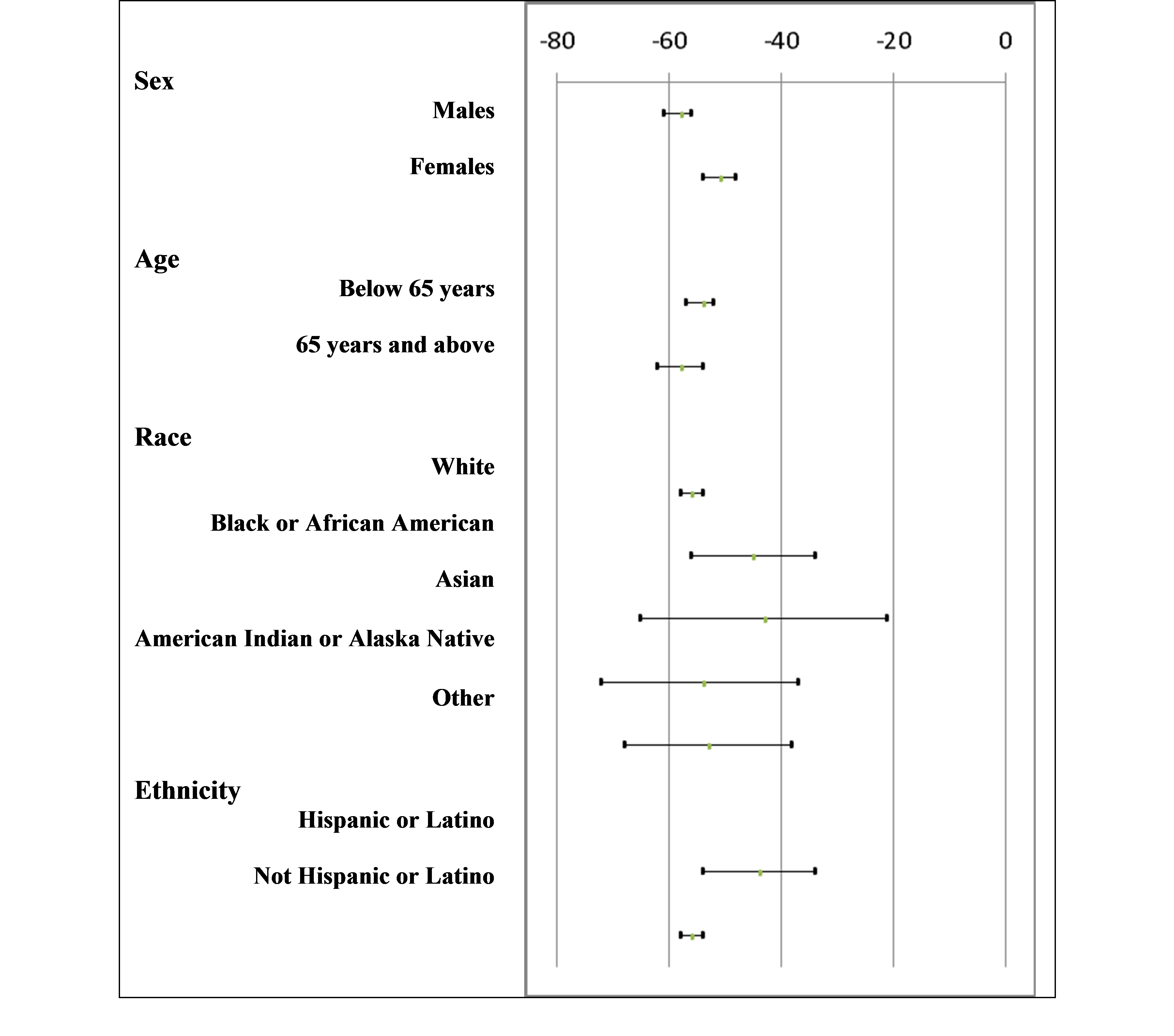
The figure below summarizes the primary efficacy endpoint, the mean percent change in LDL-C from baseline to week 24, by sex, age, race, and ethnicity. Data are provided combining the five placebo-controlled trials to allow for the largest possible sample sizes in each subgroup and since comparisons of the treatment effect across subgroups were consistent across trials. Statistical tests assessing whether the treatment effect varied across subgroups are provided.
Figure 4: Difference (95% Confidence Interval) in Average Percent LDL-C Change at Week 24 (Praluent minus placebo); Trials 1 to 5 Combined (N=3499)
P-value for statistical test measuring whether the treatment effect differs across subgroups (i.e., p-value for test of treatment-by-subgroup interaction) for studies 1 to 5 combined: Sex: 0.001; Age: 0.14; Race: 0.25; Ethnicity: 0.02
Source: FDA Statistical Review and Evaluation for Drug Snapshot (PDF - 276 KB)
What are the possible side effects?
The most common side effects are common cold, injection site reactions, and flu.
PRALUENT may cause serious allergic reactions that require being hospitalized.
What are the possible side effects?
The table below summarizes adverse reactions from the nine placebo-controlled trials that were pooled for an evaluation of safety. The population represented is the Safety population, which includes any patient who received at least one dose of trial drug (Praluent or placebo).
Table 3. Adverse Reactions Occurring in Greater Than or Equal to 2% of PRALUENT-Treated Patients and More Frequently than with Placebo
| Adverse Reactions | Placebo (N=1276) |
PRALUENTa (N=2476) |
|---|---|---|
| Nasopharyngitis | 11.1% | 11.3% |
| Injection site reactionsb | 5.1% | 7.2% |
| Influenza | 4.6% | 5.7% |
| Urinary tract infection | 4.6% | 4.8% |
| Diarrhea | 4.4% | 4.7% |
| Bronchitis | 3.8% | 4.3% |
| Myalgia | 3.4% | 4.2% |
| Muscle spasms | 2.4% | 3.1% |
| Sinusitis | 2.7% | 3.0% |
| Cough | 2.3% | 2.5% |
| Contusion | 1.3% | 2.1% |
| Musculoskeletal pain | 1.6% | 2.1% |
a 75 mg every 2 weeks and 150 mg every 2 weeks combined
b includes erythema/redness, itching, swelling, pain/tenderness
Source: PRALUENT Prescribing Information, Section 6, Table 1
Were there any differences in side effects among sex, race and age?
Subgroup analyses were conducted for sex, race, and age.
- Sex: The risk of side effects was similar in men and women.
- Race: The majority of patients in the trials were white. Differences in side effects among races could not be determined.
- Age: The risk of side effects was similar in all age groups studied.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes injection site reactions in nine placebo-controlled trials by subgroups. The population represented is the Safety population, which includes any patient who received at least one dose of trial drug (Praluent or placebo).
Table 4. Subgroup Analysis of Adverse Event-Injection Site Reaction
| Subgroup | PRALUENT (N=2476) |
Placebo (N=1276) |
Hazard Ratio | 95% CI | |||
|---|---|---|---|---|---|---|---|
| n (%) | Total, N | n (%) | Total, N | LL | UL | ||
| Any Injection Site Reaction | 179 (7.2) | 2476 | 65 (5.1) | 1276 | 1.48 | 1.12 | 1.97 |
| Sex | |||||||
| Male | 91 (6.1) | 1482 | 29 (3.8) | 763 | 1.68 | 1.1 | 2.55 |
| Female | 88 (8.9) | 994 | 36 (7.0) | 513 | 1.27 | 0.86 | 1.88 |
| Age Group | |||||||
| below 65 years | 142 (8.5) | 1671 | 43 (4.9) | 878 | 1.85 | 1.32 | 2.62 |
| 65 years and above | 37 (4.6) | 805 | 22 (5.5) | 398 | 0.84 | 0.49 | 1.42 |
| Race | |||||||
| White | 167 (7.5) | 2232 | 59 (5.2) | 1136 | 1.5 | 1.12 | 2.03 |
| Black or African American | 5 (5.0) | 101 | 3 (5.3) | 57 | 0.73 | 0.16 | 3.38 |
| Asian | 4 (5.3) | 75 | 1 (2.7) | 37 | 2 | 0.22 | 17.9 |
| American Indian or Alaska Native | 0 | 32 | 1 (5.3) | 19 | NE | NE | NE |
| Native Hawaiian or Other Pacific Islander | 0 | 0 | 0 | 2 | NE | NE | NE |
| Other | 3 (8.3) | 36 | 1 (4.0) | 25 | 1.88 | 0.19 | 18.94 |
N=not evaluated
Source: Company Clinical Trial Data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved PRALUENT based on evidence including that from nine clinical trials of 3752 patients.
The figure below summarizes how many men and women were in these clinical trials.
Figure 1. Baseline Demographics by Sex
Source: Company Clinical Data
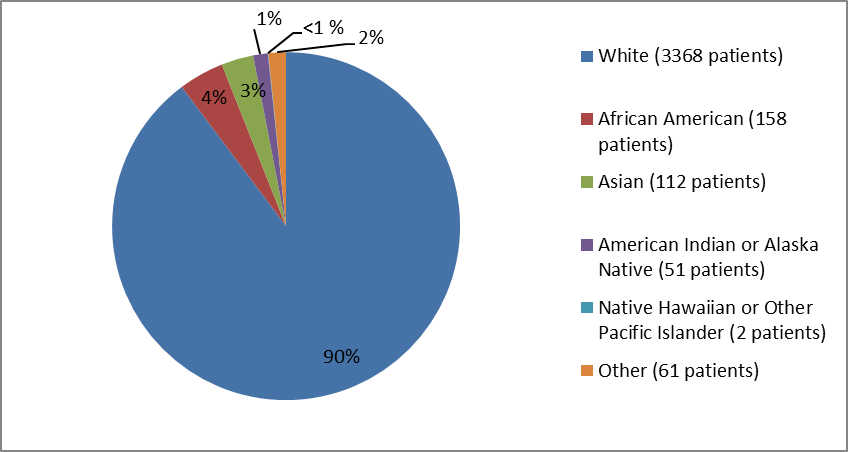
The figure and table below summarize the percentage of patients by race in these clinical trials.
Figure 2. Baseline Demographics by Race
Source: Company Clinical Data
Table 1. Baseline Demographics by Race
| Race | Number of Patients | Percentage of Patients |
|---|---|---|
| White | 3368 | 90% |
| Black or African American | 158 | 4% |
| Asian | 112 | 3% |
| American Indian or Alaska Native | 51 | 1% |
| Native Hawaiian or Other Pacific Islander | 2 | <> |
| Other | 61 | 2% |
Source: Company Clinical Data
The figure below summarizes the percentage of patients by age in the clinical trials.
Figure 3. Baseline Demographics by Age
Source: Company Clinical Data
Who participated in the trials?
The table below summarizes baseline demographics for a pool of nine placebo-controlled trials (safety population).
Table 5. Baseline Demographics
| Demographic Parameters | PRALUENT (N=2476) n (%)* |
Placebo (N=1276) n (%) |
Total(N=3752) n (%) |
|---|---|---|---|
| Sex | |||
| Male | 1482 (60) | 763 (60) | 2245 (60) |
| Female | 994 (40) | 513 (40) | 1507 (40) |
| Age | |||
| Mean years (SD) | 58.6 (11.6) | 58.5 (11.3) | 58.6 (11.4) |
| Median (years) | 60 | 59 | 60 |
| Min, Max (years) | 18, 89 | 19, 87 | 18, 89 |
| Age Group | |||
| below 65 years | 1671 (68) | 878 (69) | 2549 (68) |
| 65 years and above | 805 (32) | 398 (31) | 1203 (32) |
| Race | |||
| White | 2232 (90) | 1136 (89) | 3368 (90) |
| Black or African American | 101 (4) | 57 (4) | 158 (4) |
| Asian | 75 (3) | 37 (3) | 112 (3) |
| American Indian or Alaska Native | 32 (1) | 19 (1) | 51 (1) |
| Native Hawaiian or Other Pacific Islander | 0 | 2 (<> | 2 (<> |
| Other | 36 (2) | 25 (2) | 61 (2) |
| Ethnicity | |||
| Hispanic or Latino | 153 (6) | 77 (6) | 230 (6) |
| Not Hispanic or Latino | 2268 (94) | 1172 (94) | 3440 (94) |
| Region | |||
| United States | 725 (29) | 384 (30) | 1109 (30) |
| Rest of the world | 1751 (71) | 892 (70) | 2643 (70) |
Source: Company Clinical Trial Data
How were the trials designed?
Nine double-blind, placebo-controlled trials composed a pool for safety assessment. All patients were receiving a maximally tolerated dose of a statin, with or without other lipid modifying therapies. Patients received either PRALUENT or placebo injections subcutaneously every two weeks. Five of these trials also supported the assessment of efficacy, with the primary endpoints being the percent change in LDL-C from baseline to week 24.
How were the trials designed?
Nine double-blind, placebo-controlled trials composed a pool for safety assessment. All patients were receiving a maximally tolerated dose of a statin, with or without other lipid modifying therapies. Patients received either PRALUENT or placebo injections subcutaneously every two weeks. Five of these trials also supported the assessment of efficacy, with the primary endpoints being the percent change in LDL-C from baseline to week 24.
There were nine placebo-controlled trials that contributed to the evaluation of the side effects of PRALUENT. Five of these trials were also central to the evaluation of the benefit of PRALUENT. In each of these trials, patients were randomly assigned to receive PRALUENT or placebo injections. Neither the patients nor the health care providers knew which treatment was being given until after the trials were completed.
The benefit of PRALUENT (the percent change in LDL-C blood levels) was measured at 24 weeks.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.