Drug Trials Snapshot: ENHERTU
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the ENHERTU Package Insert for complete information.
ENHERTU (fam-trastuzumab deruxtecan-nxki)
(en HER too)
Daiichi Sankyo, Inc.
Approval date: December 20, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
ENHERTU is a drug for treatment of adults with human epidermal growth factor receptor 2 (HER2)-positive breast cancer that has spread to other parts of the body (metastatic) or cannot not be removed by surgery. It should be used in patients who have been previously treated for their metastatic disease with at least two anti-HER2 regimens.
How is this drug used?
ENHERTU is an injection. It is given by a healthcare provided directly into the vein (an intravenous infusion) once every three weeks. It takes about 90 minutes to receive the first infusion. If well tolerated, subsequent infusions take 30 minutes.
What are the benefits of this drug?
Sixty percent of 184 patients treated with ENHERTU had partial or complete shrinkage of their cancer that lasted an average of 15 months.
ENHERTU was approved under FDA’s accelerated approval program, which provides earlier patient access to a promising new drug while the company continues to conduct clinical trials to confirm that the drug works well.
What are the benefits of this drug (results of trials used to assess efficacy)?
Table 1 shows efficacy results based on the confirmed objective response rate (ORR) assessed by independent central review using RECIST v1.1 and duration of response (DOR).
Table 1. Efficacy Results by Independent Central Review in Trial 1
| Efficacy Parameter | TRIAL 1 N=184 |
|---|---|
| Confirmed ORR (95% CI) | 60.3% (52.9, 67.4) |
| Complete Response (CR) | 4.3% |
| Partial Response (PR) | 56.0% |
| Duration of Response* Median, months (95% CI)† |
14.8 (13.8, 16.9) |
ORR 95% CI calculated using Clopper-Pearson method
ORR = CR+PR
*DOR is based on median duration of follow-up of 11.1 months.
†Median DOR based on Kaplan-Meier estimate; 95% CI calculated using Brookmeyer-Crowley method
ENHERTU Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: Almost all included patients were women, therefore sex differences cannot be determined.
- Race: ENHERTU worked similarly in White and Asian patients.
- Age: ENHERTU worked similarly in patients above and below 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 2 shows the efficacy results by relevant demographic subgroups.
Table 2. Subgroup Efficacy Analyses
| Number of patients | Number of Responders | ORR (%) 95% CI |
|
|---|---|---|---|
| Overall | 184 | 111 | 60.3 (52.9, 67.5) |
| Race | |||
| White | 101 | 60 | 59.4 (49.2, 69.1) |
| Asian | 70 | 41 | 58.6 (46.2, 70.2) |
| Other | 9 | 8 | 88.9 (51.8, 99.7) |
| Age | |||
| <65 years | 140 | 83 | 59.3 (50.7, 67.5) |
| ≥65 years | 44 | 28 | 63.6 (47.8, 77.6) |
| Region | |||
| USA | 53 | 32 | 60.4 (46.0, 73.6) |
| Asian | 63 | 38 | 60.3 (47.2, 72.4) |
| EU | 68 | 41 | 60.3 (47.7, 72.0) |
ORR= objective response rate; CI=confidence interval
Adapted from FDA Review
What are the possible side effects?
ENHERTU may cause serious side effects including life threatening lung inflammation (interstitial lung disease and pneumonitis), low white blood cells (neutropenia), weakening of the heart and harm to unborn baby.
The most common side effects of ENHERTU are nausea, fatigue, vomiting, hair loss, constipation, decreased appetite, low blood cell counts, diarrhea, and cough.
What are the possible side effects (results of trials used to assess safety)?
Table 3 below summarizes common adverse reactions. This was based on safety population defined as all patients from Trials 1 and 2 who received ENHERTU 5.4 mg/kg by intravenous infusion at least once.
Table 3. Common Adverse Reactions (≥10% All Grades or ≥2% Grades 3 or 4) in Patients in Combined Trials 1 and 2
| Adverse Reactions | ENHERTU N=234 |
|
|---|---|---|
| All Grades % |
Grades 3 or 4 % |
|
| Gastrointestinal Disorders | ||
| Nausea | 79 | 7 |
| Vomiting | 47 | 3.8 |
| Constipation | 35 | 0.9 |
| Diarrhea | 29 | 1.7 |
| Abdominal paina | 19 | 1.3 |
| Stomatitisb | 14 | 0.9 |
| Dyspepsia | 12 | 0 |
| General Disorders and Administration Site Conditions | ||
| Fatiguec | 59 | 6 |
| Skin and Subcutaneous Tissue Disorders | ||
| Alopecia | 46 | 0.4d |
| Rashe | 10 | 0 |
| Metabolism and Nutrition Disorders | ||
| Decreased appetite | 32 | 1.3 |
| Hypokalemia | 12 | 3.4 |
| Blood and Lymphatic System Disorders | ||
| Anemiaf | 31 | 7 |
| Neutropeniag | 29 | 16 |
| Leukopeniah | 22 | 6 |
| Thrombocytopeniai | 20 | 3.4 |
| Respiratory, Thoracic and Mediastinal Disorders | ||
| Cough | 20 | 0 |
| Dyspnea | 13 | 1.3 |
| Epistaxis | 13 | 0 |
| Interstitial lung diseasej | 9 | 2.6k |
| Nervous System Disorders | ||
| Headachel | 19 | 0 |
| Dizziness | 10 | 0 |
| Infections and Infestation | ||
| Upper respiratory tract infectionm | 15 | 0 |
| Investigations | ||
| Aspartate aminotransferase increased | 14 | 0.9 |
| Alanine aminotransferase increased | 10 | 0.9 |
| Eye Disorders | ||
| Dry eye | 11 | 0.4n |
Events were graded using NCI-CTCAE version 4.03. N=number of patients exposed; PT = preferred term.
Percentages were calculated using the number of patients in the Safety Analysis Set as the denominator.
a Grouped term of abdominal pain includes PTs of abdominal discomfort, gastrointestinal pain, abdominal pain, abdominal pain lower, and abdominal pain upper.
b Grouped term of stomatitis includes PTs of stomatitis, aphthous ulcer, mouth ulceration, oral mucosa erosion, and oral mucosa blistering. One Grade 1 event of aphthous ulcer was not included in the summary of grouped term stomatitis (from DESTINY-Breast01).
c Grouped term of fatigue includes PTs of fatigue and asthenia.
d This Grade 3 event was reported by the investigator. Per NCI-CTCAE v.4.03, the highest NCI-CTCAE grade for alopecia is Grade 2.
e Grouped term of rash includes PTs of rash, rash pustular, rash maculo-papular.
f Grouped term of anemia includes PTs of anemia, hemoglobin decreased, hematocrit decreased, and red blood cell count decreased.
g Grouped term of neutropenia includes PTs of neutropenia and neutrophil count decreased.
h Grouped term of leukopenia includes PTs of leukopenia, lymphopenia, and white blood cell count decreased.
i Grouped term of thrombocytopenia includes PTs of thrombocytopenia and platelet count decreased.
j Interstitial lung disease includes events that were adjudicated as ILD: pneumonitis, interstitial lung disease, respiratory failure, organizing pneumonia, acute respiratory failure, lung infiltration, lymphangitis, alveolitis.
k All events had fatal outcomes (n=6).
l Grouped term of headache includes PTs headache, sinus headache, and migraine.
m Grouped term of upper respiratory tract infection includes PTs influenza, influenza like illness, upper respiratory tract infection.
n This Grade 4 event was reported by the investigator. Per NCI-CTCAE v.4.03, the highest NCI-CTCAE grade for dry eye is Grade 3.
ENHERTU Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: Almost all included patients were women therefore sex differences cannot be determined.
- Race: Occurrence of side effects was similar in White and Asian patients.
- Age: The occurrence of overall side effects was similar in patients above and below 65 years of age. There was a higher incidence of more severe (Grade 3-4) side effects observed in patients aged 65 years or older as compared to younger patients.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Analysis of side effects by subgroup was limited to age and race due to the fact that all but one patient were women.
Table 4. Summary of Treatment-Emergent Adverse Events (TEAEs) by Race
| Adverse Event | ENHERTU N=234 |
|
|---|---|---|
| White N=119 |
Asian N=97 |
|
| Any TEAEs, n (%) | 118 (99.2) | 97 (100) |
| Grade ≥3 or 4, n (%) | 61 (51.3) | 45 (46.4) |
| SAEs, n (%) | 29 (24.4) | 16 (16.5) |
Table 5. Summary of Treatment-Emergent Adverse Events (TEAEs) by Age
| Adverse Event | ENHERTU N=234 |
|
|---|---|---|
| <65y N=173 |
≥65y N=61 |
|
| Any TEAEs, n (%) | 172 (99.4) | 61 (100) |
| Grade ≥3, n (%) | 82 (47.4) | 35 (57.4) |
| SAEs, n (%) | 33 (19.1) | 14 (23) |
SAEs=serious adverse events
FDA Review and Clinical Trial Report
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved ENHERTU based on evidence from two clinical trials (Trial 1 NCT03248492/ and Trial 2/NCT02564900) of 234 patients with HER2-positive metastatic breast cancer. The trials were conducted at 86 sites in Europe, United States, Japan and South Korea.
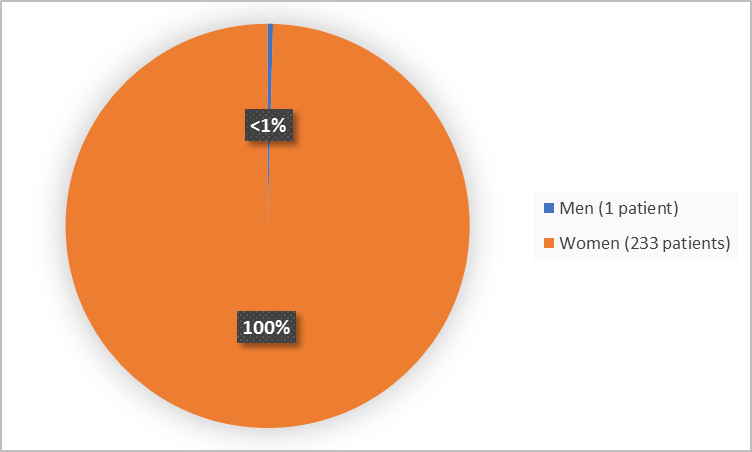
Figure 1 summarizes by sex how many patients were in the clinical trials 1 and 2 combined.
Figure 1. Baseline Demographics by Sex
FDA Review
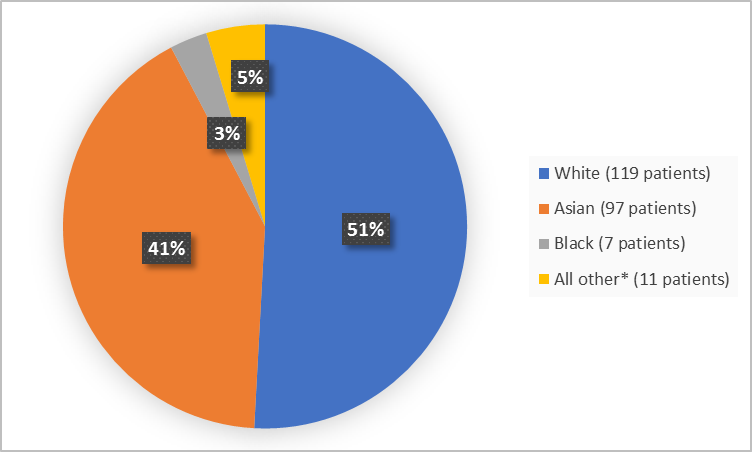
Figure 2 summarizes patients by race in the clinical trials.
Figure 2. Baseline Demographics by Race
*includes American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, Other, and missing
FDA Review
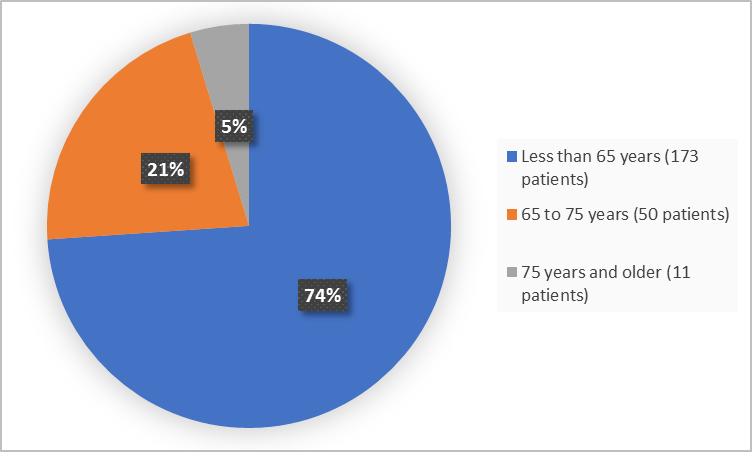
Figure 3 summarizes patients by age in the clinical trials.
Figure 3. Baseline Demographics by Age
FDA Review
Who participated in the trials?
The demographic characteristics of safety population are summarized in Table 6.
Table 6. Baseline Demographic Characteristics (safety population)
| Demographic Characteristic | Trial 1 (N = 184) |
Trial 2 (N = 50) |
Total (N=234) |
|---|---|---|---|
| Sex, n (%) | |||
| Men | 0 | 1 (2.0) | 1 (0.4) |
| Women | 184 (100.0) | 49 (98.0) | 233 (99.6) |
| Race, n (%) | |||
| White | 101 (54.9) | 18 (36.0) | 119 (50.9) |
| Black or African American | 4 (2.2) | 3 (6.0) | 7 (3) |
| Asian | 70 (38.0) | 27 (54.0) | 97 (41.5) |
| American Indian or Alaska Native | 1 (0.5) | 1 (2.0) | 2 (0.9) |
| Native Hawaiian or Other Pacific Islander | 1 (0.5) | 0 | 1 (0.4) |
| Other | 3 (1.6) | 1 (2.0) | 4 (1.7) |
| Missing | 4 (2.2) | 0 | 4 (1.7) |
| Age (years) | |||
| Mean (Std Dev) | 56.0 (11.72) | 56.6 (12.05) | 56.1 (11.77) |
| Median | 55.0 | 58.0 | 56 |
| Range | 28, 96 | 28, 77 | 28, 96 |
| Age Group, n (%) | |||
| <65 years | 140 (76.1) | 33 (66.0) | 173 (73.9) |
| ≥65 years | 44 (23.9) | 17 (34.0) | 61 (26.1) |
| <75 years | 175 (95.1) | 48 (96.0) | 223 (95.3) |
| ≥75 years | 9 (4.9) | 2 (4.0) | 11 (4.7) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 10 (5.4) | 3 (6.0) | 13 (5.6) |
| Not Hispanic/Not Latino | 141 (76.6) | 26 (52.0) | 167 (71.4) |
| Not Applicable* | 32 (17.4) | 0 | 32 (13.7) |
| Missing | 1 (0.5) | 21 (42.0) | 22 (9.4) |
| Region, n (%) | |||
| US | 53 (28.8) | 29 (58.0) | 82 (35) |
| Japan | 30 (16.3) | 21 (42.0) | 51 (21.8) |
| Korea | 33 (17.9) | 0 | 33 (14.1) |
| Belgium | 7 (3.8) | 0 | 7 (3) |
| France | 19 (10.3) | 0 | 19 (8.1) |
| Italy | 9 (4.9) | 0 | 9 (3.8) |
| Spain | 21 (11.4) | 0 | 21 (9) |
| United Kingdom | 12 (6.5) | 0 | 12 (5.1) |
*Ethnicity was not required to be collected in all countries per local authority
FDA Review
How were the trials designed?
There were two trials that provided data for ENHERTU approval. Both trials enrolled patients with HER2-positive, metastatic breast cancer who have been treated presviously for their metastatic disease.
In Trial 1 patients received ENHERTU by intravenous infusion every three weeks and tumor imagining was obtained every six weeks. The treatment continued until the disease progressed or the side effects became too toxic.
The trial measured the percentage of patients with partial or complete cancer shrinkage and how long that shrinkage lasted. It also provided the data about the side effects.
Trial 2 collected the data on ENHERTU side effects from patients who received the same dosing regimen as the ones from Trial 1.
How were the trials designed?
There were two trials that provided data for approval of ENHERTU.
Trial 1 was an open-label, multicenter, clinical trial. All patients were women with HER2-positive unresectable and/or metastatic breast cancer who had received two or more prior anti-HER2 therapies. Patients received ENHERTU 5.4 mg/kg by intravenous infusion every 3 weeks until unacceptable toxicity or disease progression. Tumor assessments were performed at screening and every 6 weeks thereafter using computed tomography (CT) or magnetic resonance imaging (MRI).
The major efficacy outcomes were ORR assessed by independent central review using RECIST v1.1 and DOR.
Trial 2 was a non-randomized, open-label, multiple dose, multicenter trial that provided additional safety data from patients with unresectable or metastatic HER2-positive breast cancer who received at least one dose of ENHERTU 5.4 mg/kg.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION