Drug Trial Snapshot: ORGOVYX
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the ORGOVYX Package Insert for complete information.
ORGOVYX (relugolix)
(or-GO-vix)
Myovant Sciences, Inc.
Approval date: December 18, 2020
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
ORGOVYX is a drug for the treatment of adults with advanced prostate cancer.
How is this drug used?
ORGOVYX is a tablet. Three tablets (total of 360 mg) are taken on the first day of treatment followed by one tablet once daily.
What are the benefits of this drug?
Because prostate cancer cells use testosterone for growth, the trial tested the ability of ORGOVYX to keep testosterone level as low as after castration.
In this trial, 97% of all patients treated with ORGOVYX reached and maintained low testosterone levels equivalent to castration level.
What are the benefits of this drug (results of trials used to assess efficacy)?
The primary efficacy outcome measure of the clinical trial was medical castration rate defined as achieving and maintaining serum testosterone suppression to castrate levels (< 50 ng/dL) by Day 29 through 48 weeks of treatment.
The efficacy results are shown in Table 1.
Table 1. Medical Castration Rates (Testosterone Concentrations < 50 ng/dL) from Day 29 through Week 48 in ORGOVYX-Treated Patients
|
|
ORGOVYX |
|---|---|
|
Castration Rate (95% CI)b
|
96.7% |
a Two patients did not receive the study treatment and were not included.
b Kaplan-Meier estimates within group.
Adapted from ORGOVYX Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: All the patients were men since ORGOVYX is for the treatment of prostate cancer.
- Race: ORGOVYX worked similarly in all tested groups.
- Age: ORGOVYX worked similarly in patients below or above 75 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The table below presents analyses of primary endpoint by demographic subgroups.
Table 2. Subgroup Analysis for Medical Castration Rate of ORGOVYX-Treated Patients
|
Demographic Subgroup |
Castration Rate |
|---|---|
|
Overall |
96.7% (94.9%, 97.9%) |
|
Race |
|
|
White |
96.9% (94.6%, 98.3%) |
|
Black or African American |
93.3% (75.9%, 98.3%) |
|
Other |
96.6 % (92.1%, 98.6%) |
|
Age |
|
|
≤75 years |
95.6% (93.1%, 97.2%) |
|
>75 years |
99.4 % (95.9%, 99.9%) |
CI=confidence interval
Adapted from FDA Review
What are the possible side effects?
ORGOVYX may cause serious side effects including heart rhythm problems because of changes in electrical activity (QT prolongation). ORGOVIX may cause harm to an unborn baby.
The most common side effects of ORGOVYX include hot flushes, increased blood sugar levels, increased blood fat (triglyceride) levels, muscle and joint pain, low red blood cell count (anemia), increased liver enzymes, tiredness, constipation and diarrhea.
What are the possible side effects (results of trials used to assess safety)?
The tables below show the side effects and laboratory abnormalities noted in patients who received at least one dose of study treatment.
Table 3. Adverse Reactions Occurring in ≥10% of Patients with Advanced Prostate Cancer Who Received ORGOVYX
|
Adverse Reaction
|
ORGOVYX |
Leuprolide Acetate |
||
|---|---|---|---|---|
|
All Grades |
Grade 3-4 |
All Grades |
Grade 3-4 |
|
|
Vascular disorders |
||||
|
Hot flush |
54 |
0.6 |
52 |
0 |
|
Musculoskeletal and connective tissue disorders |
||||
|
Musculoskeletal paina |
30 |
1.1 |
29 |
1.6 |
|
General |
||||
|
Fatigueb |
26 |
0.3 |
24 |
0 |
|
Gastrointestinal disorders |
||||
|
Diarrheac |
12 |
0.2 |
7 |
0 |
|
Constipation |
12 |
0 |
10 |
0 |
a Includes arthralgia, back pain, pain in extremity, musculoskeletal pain, myalgia, bone pain, neck pain, arthritis, muculoskeletal stiffness, non-cardiac chest pain, musculoskeletal chest pain, spinal pain, and musculoskeletal discomfort.
b Includes fatigue and asthenia
c Includes diarrhea and colitis
Table 4. Select Laboratory Abnormalities (≥ 15%) That Worsened from Baseline in Patients with Advanced Prostate Cancer Who Received ORGOVYX
|
Laboratory Test |
ORGOVYXa |
Leuprolide Acetatea |
||
|---|---|---|---|---|
|
All Grades |
Grade 3-4 |
All Grades |
Grade 3-4 |
|
|
Chemistry |
||||
|
Glucose increased |
44 |
2.9 |
54 |
6 |
|
Triglycerides increased |
35 |
2 |
36 |
0.7 |
|
Alanine aminotransferase |
27 |
0.3 |
28 |
0 |
|
Aspartate aminotransferase |
18 |
0 |
19 |
0.3 |
|
Hematology |
||||
|
Hemoglobin decreased |
28 |
0.5 |
29 |
0.7 |
a The denominator used to calculate the rate varied from 611 to 619 in the ORGOVYX arm and from 301 to 306 in the leuprolide arm based on the number of patients with a baseline value and at least one post-treatment value.
ORGOVYX Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: All the patients were men since ORGOVYX is for the treatment of prostate cancer.
- Race: The occurrence of side effects was similar in all tested groups.
- Age: The occurrence of side effects was similar in patients below or above 75 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The tables below show frequent adverse reactions by race and age subgroups noted in the clinical trial.
Table 5. Treatment-Emergent Adverse Events (TEAE) by Race
|
TEAE
|
White |
Asian |
||||||
|---|---|---|---|---|---|---|---|---|
|
ORGOVYX |
Leuprolide Acetate |
OROVYX |
Leuprolide Acetate |
|||||
|
All Grades |
Grade 3-4 |
All Grades |
Grade 3-4 |
All Grades |
Grade 3-4 |
All Grades |
Grade 3-4 |
|
|
Any TEAE |
94 |
17 |
96 |
18 |
88 |
16 |
90 |
20 |
|
Vascular disorders |
62 |
2 |
67 |
2 |
40 |
2 |
31 |
0 |
|
Hot flush |
58 |
0 |
58 |
0 |
35 |
1 |
27 |
0 |
|
Hypertension |
7 |
1 |
15 |
1 |
5 |
2 |
4 |
0 |
|
Musculoskeletal and connective tissue disorders |
40 |
2 |
38 |
2 |
30 |
2 |
31 |
3 |
|
Arthralgia |
12 |
0.2 |
10 |
0 |
10 |
1 |
6 |
0 |
|
Back pain |
9 |
1 |
9 |
1 |
6 |
0 |
11 |
1 |
|
General disorders and administration |
37 |
1 |
4 |
1 |
28 |
0 |
23 |
0 |
|
Fatigue |
24 |
0.2 |
23 |
0 |
12 |
0 |
7 |
0 |
|
Gastrointestinal disorders |
34 |
3 |
35 |
3 |
39 |
1 |
32 |
4 |
|
Diarrhea |
13 |
0 |
8 |
0 |
6 |
0 |
1 |
0 |
|
Constipation |
10 |
0 |
11 |
0 |
21 |
0 |
7 |
0 |
Table 6. Treatment-Emergent Adverse Events (TEAE) by Age
|
TEAE |
≤75 years |
>75 years |
||||||
|---|---|---|---|---|---|---|---|---|
|
OROVYX |
Leuprolide Acetate |
OROVYX |
Leuprolide Acetate |
|||||
|
All Grades |
Grade 3-4 |
All Grades |
Grade 3-4 |
All Grades |
Grade 3-4 |
All Grades |
Grade 3-4 |
|
|
Any TEAE |
92 |
16 |
92 |
18 |
94 |
19 |
97 |
17 |
|
Vascular disorders |
61 |
2 |
59 |
1 |
54 |
3 |
58 |
1 |
|
Hot flush |
56 |
1 |
51 |
0 |
50 |
0 |
52 |
0 |
|
Musculoskeletal and connective tissue disorders |
37 |
2 |
39 |
3 |
39 |
2.2 |
25 |
1 |
|
Back pain |
8 |
0.2 |
11 |
1 |
9 |
1 |
3 |
0 |
|
General disorders and administration site conditions |
6 |
1 |
38 |
1 |
33 |
1 |
36 |
1 |
|
Fatigue |
24 |
1 |
18 |
0 |
16 |
0 |
19 |
0 |
|
Gastrointestinal disorders |
35 |
2 |
35 |
2 |
39 |
1 |
33 |
3 |
|
Diarrhea |
13 |
0 |
7 |
0 |
11 |
0 |
6 |
0 |
|
Constipation |
10 |
0 |
10 |
0 |
18 |
0 |
8 |
0 |
FDA Review
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved ORGOVYX based on evidence from a clinical trial ((NCT03085095) of 930 patients 48 to 97 years old with advanced prostate cancer. The trial was conducted at 155 sites in the United States, Canada, and countries in South America, Europe and the Asia Pacific region.
Figures below summarize how many were in the clinical trial by sex, race, age and ethnicity.
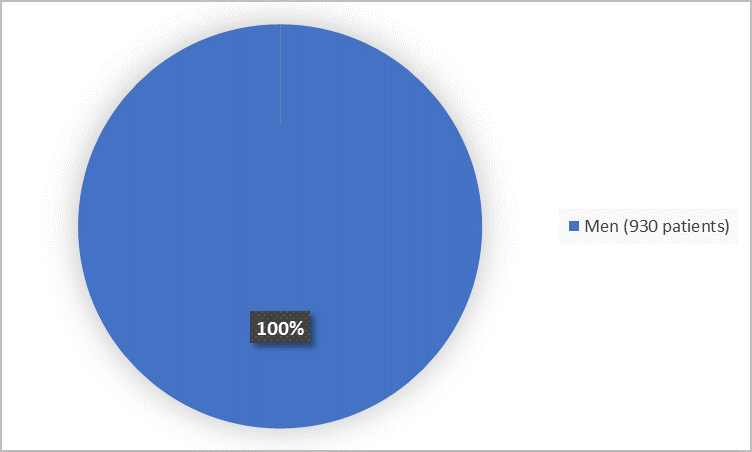
Figure 1. Demographics by Sex
FDA Review
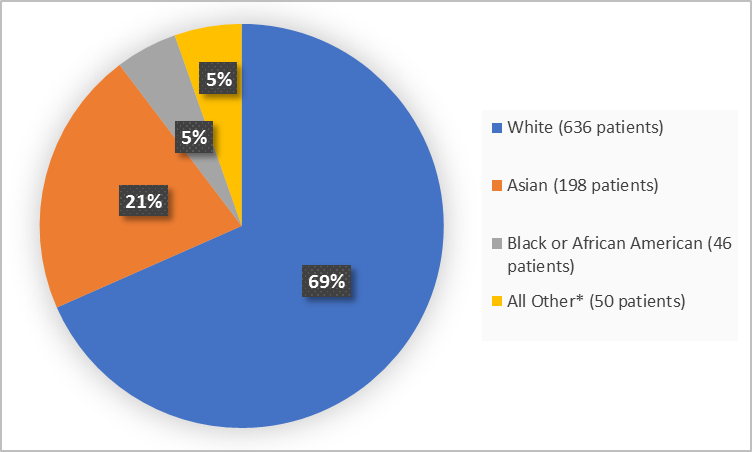
Figure 2. Demographics by Race
*Includes Other, Multiple and Not Reported
FDA Review
Figure 3. Demographics by Age
FDA Review
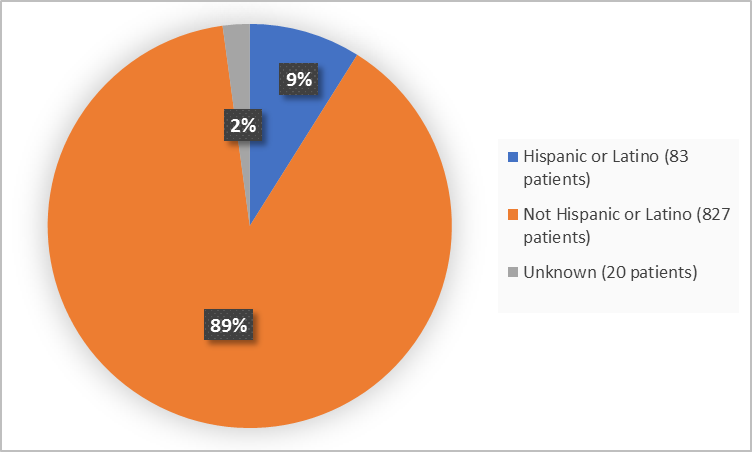
Figure 4. Demographics by Ethnicity
FDA Review
Who participated in the trials?
The table below presents the trial demographics
Table 7. Trial Demographics
|
Demographic Category
|
ORGOVYX |
Leuprolide acetate |
Total |
|---|---|---|---|
|
Sex |
|||
|
Men |
622 (100%) |
308 (100%) |
930 (100%) |
|
Race |
|||
|
Asian |
127 (20.4%) |
71 (23.1%) |
198 (21.3%) |
|
Black or African American |
30 (4.8%) |
16 (5.2%) |
46 (4.9%) |
|
White |
434 (69.8%) |
202 (65.6%) |
636 (68.4%) |
|
Other |
8 (1.3%) |
7 (2.3%) |
15 (1.6%) |
|
Multiple |
11 (1.8%) |
4 (1.3%) |
15 (1.6%) |
|
Not Reported |
12 (1.9%) |
8 (2.6%) |
20 (2.2%) |
|
Age |
|||
|
Mean (SD) |
71.2 (7.75) |
71.0 (8.03) |
71.1 (7.84) |
|
Median |
72.0 |
71.0 |
71.0 |
|
Min, Max |
48, 91 |
47, 97 |
47, 97 |
|
Age category |
|||
|
<65 years |
118 (19.9%) |
55 (17.6%) |
173 (18.6%) |
|
65-75 years |
326 (52.4%) |
165 (53.6%) |
491 (53.8%) |
|
> 75 years |
178 (28.6%) |
88 (28.6%) |
266 (28.6%) |
|
Ethnicity |
|||
|
Not Hispanic or Latino |
558 (89.7%) |
269 (87.3%) |
827 (88.9%) |
|
Hispanic or Latino |
52 (8.4%) |
31 (10.1%) |
83 (8.9%) |
|
Unknown |
12 (1.9%) |
8 (2.6%) |
20 (2.2%) |
|
Geographic region |
|||
|
USA |
156 (25%) |
69 (22.4%) |
225 (24.2%) |
|
Canada |
26 (4.2%) |
18 (5.8) |
44 (4.7%) |
|
South America |
34 (5.5%) |
19 (6.2%) |
53 (5.7%) |
|
Europe |
247 (39.7%) |
122 (39.6%) |
369 (39.7%) |
|
Asia |
125 (20.1%) |
70 (22.7%) |
195 (21.0%) |
|
Rest of World |
34 (5.5%) |
10 (3.2%) |
44 (4.7%) |
FDA Review
How were the trials designed?
There was one trial that assessed efficacy and side effects of ORGOVYX.
All patients in the trial had advanced prostate cancer. Patients were randomly assigned to receive either one ORGOVYX tablet daily (on the first day they received three tables) or an active control (leuprolide acetate) which was given as an injection under the skin every three months. The patients and healthcare providers were aware of which treatment was being given. The treatment lasted for 48 weeks.
The efficacy of ORGOVYX was assessed by the percentage of patients who achieved and maintained low testosterone level equal to castration.
How were the trials designed?
The safety and efficacy of ORGOVYX was evaluated in one randomized, open label trial in men with advanced prostate cancer requiring at least 1 year of androgen deprivation therapy and defined as biochemical (PSA) or clinical relapse, following local primary intervention, newly diagnosed castration-sensitive metastatic disease, or advanced localized disease.
Patients were randomized 2:1 to receive either ORGOVYX tablet daily or leuprolide acetate subcutaneously every 3 months for 48 weeks.
The major efficacy outcome measure was medical castration rate, defined as achieving and maintaining serum testosterone suppression to castrate levels (< 50 ng/dL) by Day 29 through 48 weeks of treatment.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.