Drug Trial Snapshot: ONPATTRO
HOW TO USE THIS SNAPSHOT
ONPATTRO is a drug for the treatment of nerve damage in adult patients with hereditary transthyretin-mediated amyloidosis. The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the ONPATTRO Package Insert for complete information.
ONPATTRO (patisiran)
on-PAH-troh
Alnylam Pharmaceuticals, Inc.
Approval date: August 10, 2018
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
ONPATTRO is a drug for the treatment of nerve damage in adult patients with hereditary transthyretin-mediated amyloidosis. Transthyretin-mediated amyloidosis is the buildup of abnormal deposits of a substance called amyloid in the body's organs and tissues. Amyloid disrupts the function of organs and tissues.
How is this drug used?
A healthcare provider injects ONPATTRO directly into the bloodstream through a needle in the vein. This is known as an intravenous, or IV infusion. It takes about 80 minutes to receive an ONPATTRO infusion.
ONPATTRO is given once every three weeks.
The amount of drug used depends on the patient’s weight.
What are the benefits of this drug?
Compared to patients receiving placebo infusions, patients who received ONPATTRO had less symptoms of nerve damage, as well as better muscle strength and reflexes.
What are the benefits of this drug (results of trials used to assess efficacy)?
The figure below summarizes efficacy results for the evaluated patients for Trial 1. The main trial endpoint was the change from baseline to Month 18 in the modified Neurologic Impairment Score +7 (mNIS+7) including objective assessment of nerve damage.
Table 2. Clinical Primary Efficacy Results from Trial 1
|
Primary Endpointa |
Baseline, Mean (SD) |
Change from Baseline to Month 18, LS Mean (SEM) |
ONPATTRO- Placebo |
p-value |
||
|---|---|---|---|---|---|---|
|
ONPATTRO |
Placebo |
ONPATTRO |
Placebo |
|||
|
mNIS+7b |
80.9 (41.5) |
74.6 (37.0) |
‑6.0 (1.7) |
28.0 (2.6) |
‑34.0 |
p<> |
|
|
||||||
LS mean, least squares mean; SD, standard deviation; SEM, standard error of the mean; CI, confidence interval
a All endpoints analyzed using the mixed-effect model repeated measures (MMRM) method.
b A lower number indicates less impairment/fewer symptoms
ONPATTRO Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: ONPATTRO worked similarly in males and females.
- Race: The majority of patients in the trial was White. Differences in how well the drug worked among races could not be determined because of the small number of patients in other races.
- Age: ONPATTRO worked similarly in patients younger and older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The table below summarizes efficacy results by sex, race and age.
Table 3. Subgroup Analyses of the Primary endpoint, mNIS+7 (Trial 1)
|
Subgroup |
Baseline |
Change from Baseline at Month 18 LS Mean (SEM) |
ONPATTRO-Placebo Treatment Difference |
||
|---|---|---|---|---|---|
|
|
ONPATTRO |
Placebo |
ONPATTRO |
Placebo |
|
|
Sex |
|||||
|
Male |
109, 82 |
58, 78 |
-5.6 (1.9) |
29.5 (2.8) |
-35.1 (-41.8, -28.4) |
|
Female |
39, 77 |
19, 65 |
-2.2 (3.4) |
29.5 (5.5) |
-31.7 (-44.6, -18.8) |
|
Race |
|||||
|
White |
113, 81 |
50, 73 |
-6.0 (1.8) |
27.9 (3.0) |
-33.9 (-40.7, -27.1) |
|
All Others |
33, 83 |
26, 76 |
-1.9 (4.4) |
31.9 (5.0) |
-33.7 (-46.5, -21.0) |
|
Age Group |
|||||
|
< 65=""> |
86, 74 |
44, 70 |
-6.3 (2.1) |
24.3 (3.1) |
-30.6 (-38.0, -23.3) |
|
> 65 years |
62, 91 |
33, 80 |
-2.3 (2.7) |
36.3 (4.1) |
-38.5 (-48.3, -28.8) |
FDA Review
What are the possible side effects?
ONPATTRO may cause serious side effects, including infusion-related reactions and decreased vitamin A levels.
The most common side effects are upper respiratory tract infections and infusion-site reactions.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions in patients with hereditary transthyretin-mediated amyloidosis (Trial 1).
Table 4. Adverse Reactions that Occurred in at Least 5% of ONPATTRO-treated Patients and at Least 3% More Frequently than in Placebo-treated Patients (Trial 1)
|
Adverse Reaction |
ONPATTRO |
Placebo |
|---|---|---|
|
Upper respiratory tract infectionsa |
29 |
21 |
|
Infusion-related reactionb |
19 |
9 |
|
Dyspepsia |
8 |
4 |
|
Dyspneac,d |
8 |
0 |
|
Muscle spasmsc |
8 |
1 |
|
Arthralgiac |
7 |
0 |
|
Erythemac |
7 |
3 |
|
Bronchitise |
7 |
3 |
|
Vertigo |
5 |
1 |
a Includes nasopharyngitis, upper respiratory tract infection, respiratory tract infection, pharyngitis, rhinitis, sinusitis, viral upper respiratory tract infection, upper respiratory tract congestion.
b Infusion-related reaction symptoms include, but are not limited to: arthralgia or pain (including back, neck, or musculoskeletal pain), flushing (including erythema of face or skin warm), nausea, abdominal pain, dyspnea or cough, chest discomfort or chest pain, headache, rash, chills, dizziness, fatigue, increased heart rate or palpitations, hypotension, hypertension, facial edema.
c Not part of an infusion-related reaction.
d Includes dyspnea and exertional dyspnea.
ONPATTRO Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in males and females.
- Race: The majority of patients in the trial was White. Differences in the occurrence of side effects among races could not be determined because of the small number of patients in other races.
- Age: The occurrence of side effects was similar in patients younger and older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes the occurrence of the most common adverse reaction, upper respiratory tract infections, by subgroup.
Table 5. Pooled Subgroup Analysis of Upper Respiratory Tract Infections (Trial 1)
|
Demographic Characteristic |
ONPATTRO |
Placebo |
|---|---|---|
|
Sex |
||
|
Male |
36/109 (33) |
14/58 (24) |
|
Female |
7/39 (18) |
2/19 (11) |
|
Race |
||
|
White |
40/113 (35) |
7/50 (14) |
|
Black or African American |
1/4 (25) |
1/1 (100) |
|
Asian |
2/27(7) |
8/25 (32) |
|
Other |
0/4 (0) |
0/1 (0) |
|
Age Group |
||
|
< 65=""> |
26/86(30) |
7/44 (16) |
|
≥ 65 years |
17/62 (27) |
9/33 (27) |
Clinical Trial Data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved ONPATTRO based on evidence from one clinical trial (Trial 1/NCT01960348) of 225 patients with hereditary transthyretin-mediated amyloidosis. The trial was conducted at 44 sites in Asia, Canada, Central America, Europe, South America, and the United States.
Figure 1 summarizes how many men and women were in the clinical trial used to evaluate efficacy and safety.
Figure 1. Baseline Demographics by Sex
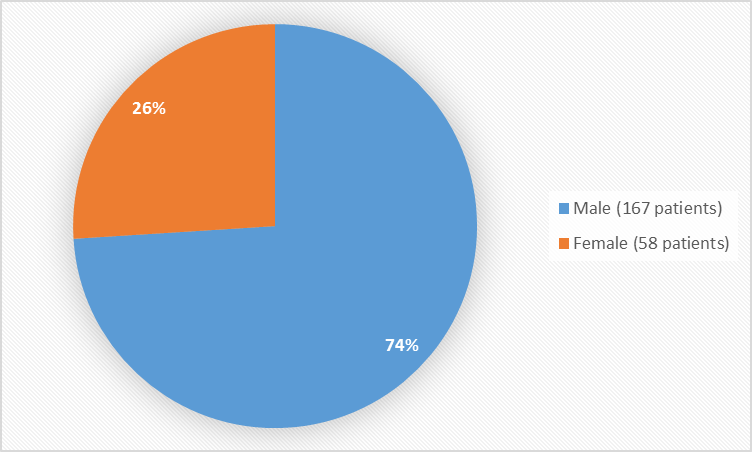
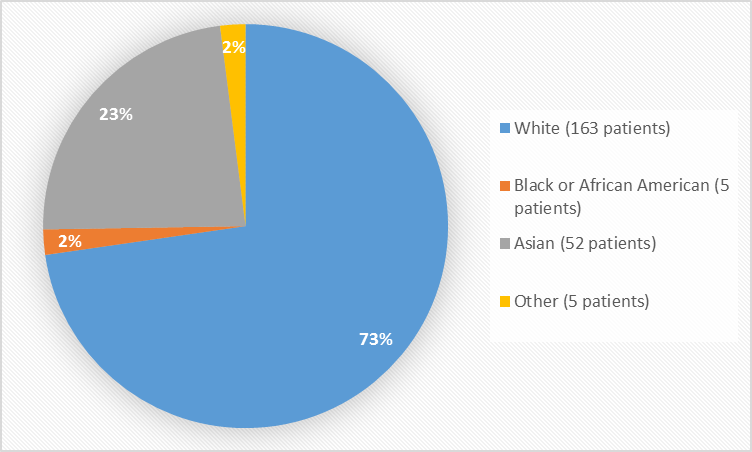
Figure 2 summarizes the percentage of patients by race in the clinical trial used to evaluate efficacy and safety.
Figure 2. Baseline Demographics by Race
FDA Review
Table 1. Demographics of Efficacy Trials by Race
|
Race |
Number of Patients |
Percentage of Patients |
|---|---|---|
|
White |
163 |
73% |
|
Black or African American |
5 |
2% |
|
Asian |
52 |
23% |
|
Other |
5 |
2% |
FDA Review
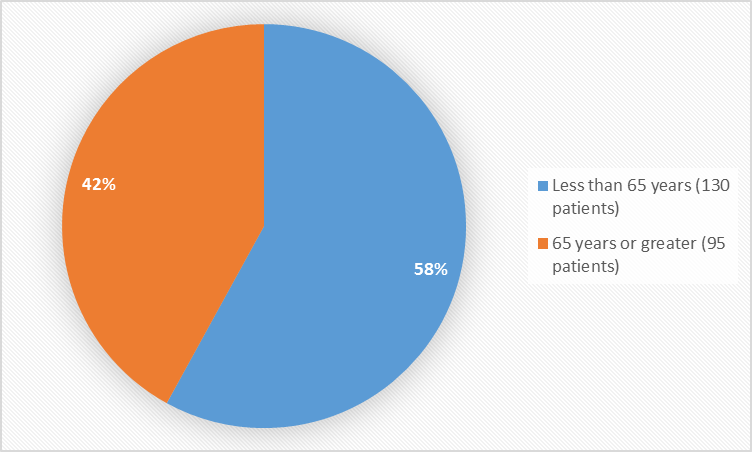
Figure 3 summarizes the percentage of patients by age enrolled in the clinical trial used to evaluate efficacy and safety.
Figure 3. Baseline Demographics by Age
FDA Review
Who participated in the trials?
The table below summarizes demographics of all patients in the clinical trial.
Table 6. Demographic Characteristics for Trial 1
|
Parameter |
ONPATTRO |
PLACEBO |
Total |
|---|---|---|---|
|
Sex, n(%) |
|||
|
Male |
109 (73.6) |
58 (75.3) |
167 (74.2) |
|
Female |
39 (26.4) |
19 (24.7) |
58 (25.8) |
|
Race, n(%) |
|||
|
White |
113 (76.4) |
50 (64.9) |
163 (72.4) |
|
Black or African American |
4 (2.7) |
1 (1.3) |
5 (2.2) |
|
Asian |
27 (18.2) |
25 (32.5) |
52 (23.1) |
|
Other |
4 (2.7) |
1 (1.3) |
5 (2.2) |
|
Age Group, n (%) |
|||
|
< 65=""> |
86 (58.1) |
44 (57.1) |
130 (57.8) |
|
65 to 74 years |
53 (35.8) |
24 (31.2) |
77 (34.2) |
|
> 75 years |
9 (6.1) |
9 (11.7) |
18 (8.0) |
|
Age in years, Mean (SD) |
59.6 (12.0) |
62.2 (10.8) |
60.5 (11.6) |
|
Ethnicity, n (%) |
|||
|
Hispanic |
17 (11.5) |
11 (14.3) |
28 (12.4) |
|
Non-Hispanic |
130 (87.8) |
65 (84.4) |
195 (86.7) |
|
Unknown |
1 (0.7) |
1 (1.3) |
2 (0.9) |
|
Region, n (%) |
|||
|
United States |
33 (22.3) |
9 (11.7) |
42 (18.7) |
|
Canada |
4 (2.7) |
1 (1.3) |
5 (2.2) |
|
Asia |
23 (15.5) |
21 (27.3) |
44 (19.6) |
|
Central and South America |
13 (8.8) |
6 (7.8) |
19 (8.4) |
|
Europe |
75 (50.7) |
40 (51.9) |
115 (51.1) |
FDA Review
How were the trials designed?
The benefits and side effects of ONPATTRO were evaluated in one clinical trial. The trial enrolled patients who had hereditary transthyretin-mediated amyloidosis. Patients were randomly assigned to receive ONPATTRO or placebo by intravenous infusion, once every 3 weeks for 18 months. All patients received medications to prevent possible allergic reactions from the infusions. Neither the patients nor the health care providers knew which treatment was being given until after the trial was completed.
Health care providers rated the change in the signs and symptoms of neuropathy (nerve damage) from baseline to Month 18 using a numerical scale. The scores for the patients receiving ONPATTRO were compared to the scores for the patients receiving placebo.
How were the trials designed?
The efficacy and safety of ONPATTRO were established in one randomized, double-blind, placebo-controlled trial. The trial evaluated ONPATTRO for the treatment of polyneuropathy of hereditary transthyretin-mediated amyloidosis in adults. Patients were randomized to receive ONPATTRO 0.3 mg/kg or placebo by intravenous infusion, once every 3 weeks for 18 months. The primary efficacy endpoint was the change from baseline to Month 18 in the modified Neurologic Impairment Score +7 (mNIS+7).
The mNIS+7 is an objective assessment of neuropathy. It measures deficits in cranial nerve function, muscle strength, reflexes, and sensation. Health care providers rate severity on a maximum possible score of 304 points. Higher scores represent greater severity of disease. The clinical meaningfulness of the objective findings on the mNIS+7 were supported by the Norfolk- Quality of Life-Diabetic Neuropathy (Norfolk-QoL-DN) scale that evaluated the functional impact of the observed changes on the mNIS+7.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.