Drug Trial Snapshot: MULPLETA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the MULPLETA Package Insert for complete information.
MULPLETA (lusutrombopag)
mul ple’ tah
Shionogi Inc.
Approval date:July 31, 2018
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
MULPLETA is a drug used to treat adults with low platelet count who are scheduled to have a medical or dental procedure that could lead to increased bleeding.
MULPLETA is to be used in patients whose low platelet count is the result of long-lasting (chronic) liver disease.
How is this drug used?
MULPLETA is a tablet that is taken 1 time per day for seven days in a row beginning 8-14 days before a scheduled procedure.
What are the benefits of this drug?
Greater proportion of patients treated with MULPLETA did not need a platelet transfusion for bleeding up to 7 days after the procedure, in comparison to patients who received placebo.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes the efficacy results for the individual trials based on the proportion of patients who did not require a platelet transfusion or any rescue procedure ((i.e., platelet preparations, other blood preparations, including red blood cells and plasma, volume expanders) for bleeding after randomization and up to 7 days following an elective procedure. Additional measure was the proportion on responders defined as patients whose platelet count reached at least 50x109/L and increased at least 20x109/L from baseline.
Table 2. Proportion of Patients Not Requiring a Platelet Transfusion Prior to Invasive Procedure and Proportion of Responders-Trial 1
Endpoint | Proportion (n/N) | Treatment Difference | |
|---|---|---|---|
MULPLETA | Placebo | ||
Not requiring platelet transfusion prior to invasive procedure* | 78% (38/49) | 13% (6/48) | 64 (49, 79) |
Responderǂ during study | 76% (37/49) | 6% (3/48) | 68 (54, 82) |
*A platelet transfusion was required if the platelet count was less than 50x109/L.
§Cochran-Mantel-Haenszel test with baseline platelet count as stratum; p value and confidence interval calculated using Wald method.
ǂPlatelet count reached at least 50x109/L and increased at least 20x109/L from baseline.
Table 3. Proportion of Patients Not Requiring Platelet Transfusion Prior to Invasive Procedure or Rescue Therapy for Bleeding Through 7 Days After Invasive Procedure and Proportion of Responders-Trial 2 (NCT02389621)
Endpoint | Proportion (n/N) | Treatment Difference | |
|---|---|---|---|
MULPLETA | Placebo | ||
Not requiring platelet transfusion prior to invasive procedure* or rescue therapy for bleeding from randomization through 7 days after invasive procedure | 65% (70/108) | 29% (31/107) | 37 (25, 49) |
Responderǂ during study | 65% (70/108) | 13% (14/107) | 52 (41, 62) |
*A platelet transfusion was required if the platelet count was less than 50x109/L. | |||
MULPLETA Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: MULPLETA worked similarly in men and women.
- Race: MULPLETA worked similarly in all races.
- Age: MULPLETA worked similarly in patients younger and older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The table below summarizes subgroup results for Trial 2 based on proportion of patients who did not require a platelet transfusion or any rescue procedure.
Since the Trial 1 enrolled smaller number of patients and all patients were Asians, subgroup analysis was not conducted.
Table 4. Summary of Subgroup Efficacy Analysis-Trial 2
Subgroup | MULPLETA | Placebo |
|---|---|---|
Sex (n/N1, %) | ||
Men | 37/65 (56.9) | 16/69 (23.2) |
Women | 33/43 (76.7) | 15/38 (39.5) |
Race(n/N1, %) | ||
White | 59/85 (69.4) | 25/86 (29.1) |
All other | 11/23 (47.8) | 6/21 (28.6) |
Age Group (n/N1, %) | ||
65> | 55/84 (65.5) | 25/88 (28.4) |
≥65 years | 15/24 (62.5) | 6/19 (31.6) |
FDA Review
What are the possible side effects?
MULPLETA may cause serious side effects such as blood clots.
The most common side effect is headache.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions in the pooled clinical trials based on safety population defined as all patients who received at least 1 dose of trial drug and had at least 1 post-dose safety assessment.
Table 9. Adverse Reactions with a Frequency ≥3% in Patients treated with MULPLETA - Pooled Data from Trials 1- 3
Adverse Reaction* | MULPLETA 3 mg | Placebo |
|---|---|---|
Headache | 5 | 4 |
*Includes treatment-emergent adverse reactions occurring at a rate higher than placebo. | ||
MULPLETA Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: The occurrence of side effects among races was similar.
- Age: The occurrence of side effects was similar in patients younger and older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes adverse events in the pooled clinical trials by subgroups (safety population).
Table 10. Subgroup Analysis of Treatment Emergent Adverse Events(TEAE) in Pooled Trials
Demographic Parameters | MULPLETA | Placebo |
|---|---|---|
Patients with Any TEAE (n/N1, %) | 112/171 (65.5) | 115/170 (67.6) |
Sex (n/N1 ,%) | ||
Men | 58/94 (61.7) | 69/107 (64.5) |
Women | 54/77 (70.1) | 46/63 (73) |
Race (n/N1 ,%) | ||
White | 40/84 (47.6) | 41/86 (47.7) |
Asian | 67/79 (84.8) | 70/80 (87.5) |
All Other | 5/8 (62.5) | 4/4 (100) |
Age Group (n/N1 ,%) | ||
59/104 (56.7) | 67/110 (60.9) | |
≥ 65 years | 53/67 (79.1) | 48/60 (80) |
Clinical Trial Data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the Clinical trials?
The FDA approved MULPLETA based on evidence from three clinical trials of 341 patients with low platelet counts because of chronic liver disease.
Trials 1 and 3 were conducted in Japan and Trial 2 (NCT02389621) was conducted at the sites located in the Europe, Asia, North America, and Australia.
Figure 1 summarizes how many men and women were in the clinical trials.
Figure 1. Baseline Demographics by Sex (safety population)

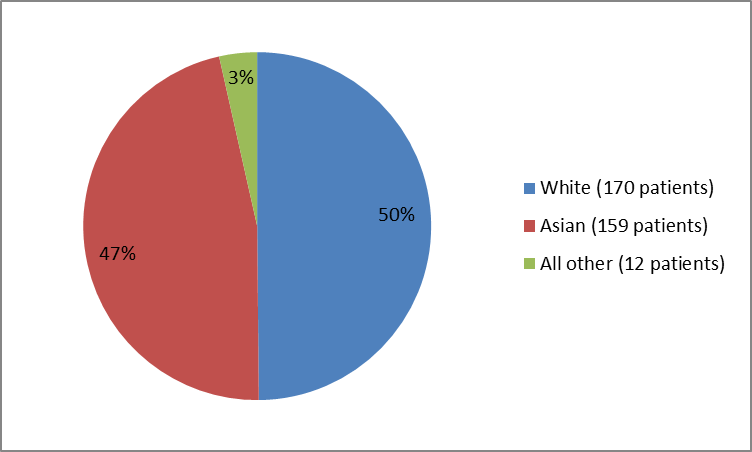
Figure 2 summarizes the percentage of patients by race in the clinical trials.
Figure 2. Baseline Demographics by Race (safety population)
Table 1. Baseline Demographics by Race
Race | Number of Patients | Percentage |
|---|---|---|
White | 170 | 50 |
Asian | 159 | 47 |
Black or African American | 1 | less than 1 |
American Indian or Alaskan Native | 2 | 1 |
Other | 9 | 3 |
FDA Review
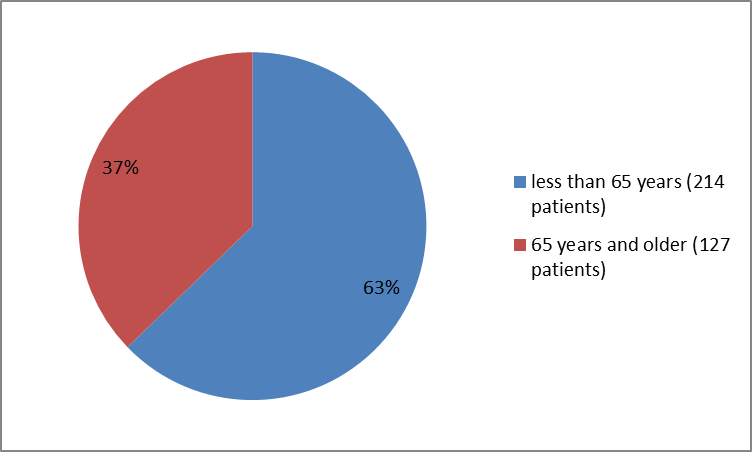
Figure 3 summarizes the percentage of patients by age in the clinical trials.
Figure 3. Baseline Demographics by Age (safety population)
FDA Review
Who participated in the trials
The table below summarizes demographics of patients that participated in all three trials.
Table 11. Patient Demographics in Combined Trials 1, 2 and 3 (Safety Population)
Demographic Parameters | MULPLETA (N=171) | Placebo | Total |
|---|---|---|---|
Sex (n, %) | |||
Men | 94 (55.0%) | 107 (62.9%) | 201 (59.5%) |
Women | 77 (45.0%) | 63 (37.1%) | 140 (41.1%) |
Race (n,%) | |||
White | 84 (49.1%) | 86 (50.6%) | 170 (49.9%) |
Asian | 79 (46.2%) | 80 (47.1%) | 159 (46.6%) |
American Indian or Alaskan Native | 2 (1.2) | 0 | 2 (0.6) |
Black or African American | 1 (0.6%) | 0 | 1 (0.3%) |
Other/Not Provided | 5 (2.9%) | 4 (2.4%) | 9 (2.6%) |
Age (years) | |||
Mean (SD) | 60.3 (11.8) | 60.4 (12.0) | 60.4 |
Median | 61.0 | 61.5 | 61.3 |
Age Group (n, %) | |||
18 – | 104 (60.8%) | 110 (64.7%) | 214 (62.8%) |
≥ 65 years | 67 (39.2%) | 60 (35.3%) | 127 (37.2%) |
Ethnicity (n, %) | |||
Hispanic | 15 (8.19%) | 12 (7.06%) | 27 (7.9%) |
Not Hispanic | 155 (93.0%) | 158 (92.9%) | 313 (91.8%) |
Unknown | 1 (0.6) | 0 | 1 (0.3) |
Region (n, %) | |||
North America | 19 (11.1%) | 11 (6.5%) | 30 (8.8%) |
Europe | 44 (25.7) | 50 (29.4) | 94 (27.6) |
Asia | 77 (45.0) | 80 (47.1) | 157 (46) |
Other | 31 (18.1) | 29 (17.1) | 60 (17.6) |
How were the trials designed?
The benefit of MULPLETA was evaluated in two trials (Trials 1 and 2) that enrolled adult patients with low platelet counts who were scheduled to have a procedure that could lead to increased bleeding. Low platelet counts were consequence of chronic liver disease.
All patients were randomly assigned to receive either MULPLETA or placebo tablets once a day for 7 consecutive days. Neither the patients nor the providers knew which treatment has been given until the end of the trials.
The benefit of MULPLETA was assessed based on the percentage of patients from two trials who did not require a platelet transfusion or any other rescue intervention for bleeding up to 7 days following the procedure.
Side effects of MULPLETA were assessed on the data from the same two trials and one additional smaller trial (Trial 3) done earlier.
How were the trials designed?
MULPLETA efficacy was assessed in two, randomized, double-blind, placebo-controlled, multi-center trials in patients with chronic liver disease who were scheduled to undergo an invasive procedure.
Patients were randomized 1:1 to receive 3 mg of MULPLETA or placebo once daily for up to 7 days.
MULPLETA efficacy was assessed on the proportion of patients who did not require a platelet transfusion prior to an elective procedure.
In addition to data from these two trials, safety assessment included an additional data from placebo-controlled phase 2 dose ranging trial M0626 conducted in Japan.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION Back to Drug Trials Snapshots