Drug Trial Snapshot: Doptelet
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the DOPTELET Package Insert for complete information.
DOPTELET (avatrombopag)
dop-TEL-et
Dova Pharmaceuticals
Approval date:May 21, 2018
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
DOPTELET is a drug used to treat adults with low platelet count who are scheduled to have a medical or dental procedure that could lead to increased bleeding.
DOPTELET is to be used in patients whose low platelet count is the result of long-lasting (chronic) liver disease.
How is this drug used?
DOPTELET is a tablet that is taken 1 time per day for five days in a row beginning 10-13 days before a scheduled procedure. The number of tables to be taken each day is based on a patient’s platelet count prior to a scheduled procedure.
What are the benefits of this drug?
Greater proportion of patients treated with DOPTELET did not need a platelet transfusion for bleeding after the procedure, in comparison to patients who received placebo.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes the efficacy results for the individual trials stratified by baseline platelet count.
The major efficacy outcome was the proportion of patients who did not require a platelet transfusion or any rescue procedure (whole blood transfusion, packed red blood cell transfusion, platelet transfusion, fresh frozen plasma, or cryoprecipitate administration, Vitamin K, desmopressin, recombinant activated factor VII, aminocaproic acid, tranexamic acid, or surgical or interventional radiology procedures performed to achieve hemostasis and control blood loss) for bleeding after randomization and up to 7 days following an elective procedure.Table 2. Proportion of Patients Not Requiring a Platelet Transfusion or Any Rescue Procedure for Bleeding by Baseline Platelet Count Cohort and Treatment Group – Trial-1 and Trial-2
|
Low Baseline Platelet Count Cohort (40>9/L) |
||||||||
|---|---|---|---|---|---|---|---|---|
|
Category |
Trial-1 |
Trial-2 |
||||||
|
DOPTELET |
Placebo (n=48) |
DOPTELET |
Placebo (n=43) |
|||||
|
Responders |
66% |
23% |
69% |
35% |
||||
|
Difference of Proportion vs. Placebob |
43% |
34% |
||||||
|
p-valued |
˂0.0001 |
0.0006 |
||||||
|
High Baseline Platelet Count Cohort (≥40 to 50>9/L) |
||||||||
|
Category |
ADAPT-1 |
ADAPT-2 |
||||||
|
DOPTELET |
Placebo (n= 34) |
DOPTELET |
Placebo (n=33) |
|||||
|
Responders |
88% |
38% |
88% |
33% |
||||
|
Difference of Proportion vs. Placebob |
50% |
55% |
||||||
|
p-valued |
˂0.0001 |
˂0.0001 |
||||||
|
a Two-sided 95% confidence interval based on normal approximation. b Difference of proportion vs. placebo = proportion of Responders for DOPTELET – proportion of Responders for placebo c 95% confidence interval calculated based on normal approximation. d By Cochran-Mantel-Haenszel Testingstratified by bleeding risk for the procedure. |
||||||||
In addition, both trials demonstrated a higher proportion of patients who achieved the target platelet count of ≥ 50 x 109/L on the day of the procedure, a secondary efficacy endpoint, in both DOPTELET-treated groups versus the placebo-treated groups for both cohorts.
DOPTELET Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: DOPTELET worked similarly in men and women.
- Race: DOPTELET worked similarly in all races.
- Age: DOPTELET worked similarly in patients younger and older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The tables below summarize primary efficacy results by sex (Tables 3 and 4), race (Tables 5 and 6) and age (Tables 7 and 8) for Trials 1 and 2 separately. All analyses were based on proportion of responders (patients who did not require a platelet transfusion or any rescue procedure).
Table 3. Summary of Proportion of Responders by Sex -Trial 1
|
Sex |
Low Baseline PLT Count Cohort |
High baseline PLT count cohort |
||
|---|---|---|---|---|
|
DOPTELET (60 mg) |
Placebo |
DOPTELET (40 mg) |
Placebo |
|
|
Men |
||||
|
Responder, n (%) |
41 (63.1) |
8 (25.0) |
33 (89.2) |
9 (37.5) |
|
95% CI |
(51.3, 74.8) |
(10.0, 40.0) |
(79.2, 99.2) |
(18.1, 56.9) |
|
95% CI of diff. |
38.1 (19.0, 57.1) |
|
51.7 (29.9, 73.5) |
|
|
Women |
||||
|
Responder, n (%) |
18 (72.0) |
3 (18.8) |
19 (86.4) |
4 (40.0) |
|
95% CI |
(54.4, 89.6) |
(0.0, 37.9) |
(72.0, 100.0) |
(9.6, 70.4) |
|
95% CI of diff. |
53.3 (27.3, 79.2) |
|
46.4 (12.8, 79.9) |
|
|
Sex |
Low Baseline PLT Count Cohort |
High Baseline PLT Count Cohort |
||
|---|---|---|---|---|
|
DOPTELET (60 mg) |
Placebo |
DOPTELET (40 mg) |
Placebo |
|
|
Men |
||||
|
Responder, n (%) |
33 (66.0) |
6 (22.2) |
28 (84.8) |
6 (35.3) |
|
95% CI |
(52.9, 79.1) |
(6.5, 37.9) |
(72.6, 97.1) |
(12.6, 58.0) |
|
95% CI of diff. |
43.8 (23.3, 64.2) |
|
49.6 (23.8, 75.4) |
|
|
Women |
||||
|
Responder, n (%) |
15 (75.0) |
9 (56.3) |
23 (92.0) |
5 (31.3) |
|
95% CI |
(56.0, 94.0) |
(31.9, 80.6) |
(81.4, 100.0) |
(8.5, 54.0) |
|
95% CI of diff. |
53.3 (27.3, 79.2) |
|
60.8 (35.7, 85.8) |
|
Table 5. Summary of Responders by Race-Trial 1
|
Race |
Low Baseline PLT Count Cohort |
High Baseline PLT Count Cohort |
||
|---|---|---|---|---|
|
DOPTELET (60 mg) |
Placebo |
DOPTELET (40 mg) |
Placebo |
|
|
White |
||||
|
Responder, n (%) |
34 (68.0) |
7 (25.0) |
30 (96.8) |
9 (47.4) |
|
95% CI |
(55.1, 80.9) |
(9.0, 41.0) |
(90.6, 100.0) |
(24.9, 69.8) |
|
95% CI of diff. |
43.0 (22.4, 63.6) |
|
49.4 (26.1, 72.7) |
|
|
Black or African American |
||||
|
Responder, n (%) |
3 (100) |
0 |
2 (100) |
0 |
|
95% CI |
(100.0, 100.0) |
|
(100.0, 100.0) |
|
|
95% CI of diff. |
|
|
|
|
|
Asian |
||||
|
Responder, n (%) |
18 (56.3) |
3 (16.7) |
18 (75.0) |
4 (26.7) |
|
95% CI |
(39.1, 73.4) |
(0.0, 33.9) |
(57.7, 92.3) |
(4.3, 49.0) |
|
95% CI of diff. |
39.6 (15.3, 63.9) |
|
48.3 (20.0, 76.6) |
|
Table 6. Summary of Proportion of Responders by Race-Trial 2
|
Race |
Low Baseline PLT Count Cohort |
High baseline PLT count cohort |
||
|---|---|---|---|---|
|
DOPTELET (60 mg) |
Placebo |
DOPTELET (40 mg) |
Placebo |
|
|
White |
||||
|
Responder, n (%) |
27 (67.5) |
10 (37.0) |
33 (82.5) |
9 (37.5) |
|
95% CI |
(53.0, 82.0) |
(18.8, 55.3) |
(70.7, 94.3) |
(18.1, 56.9) |
|
95% CI of diff. |
30.5 (7.2, 53.8) |
|
45.0 (22.3, 67.7) |
|
|
Black or African American |
||||
|
Responder, n (%) |
2 (100) |
1 (50.0) |
2 (100) |
0 |
|
95% CI |
(100.0, 100.0) |
(0.0, 100.0) |
(100.0, 100.0) |
|
|
95% CI of diff. |
50.0 (-19.3, 100.0) |
|
|
|
|
Asian |
||||
|
Responder, n (%) |
17 (68.0) |
1 (10.0) |
12 (100) |
2 (25.0) |
|
95% CI |
(49.7, 86.3) |
(0.0, 28.6) |
(100.0, 100.0) |
(0.0, 55.0) |
|
95% CI of diff. |
58.0 (31.9, 84.1) |
|
75.0 (45.0, 100.0) |
|
Table 7. Summary of Proportion of Responders by Age Group-Trial 1
|
Low Baseline PLT Count Cohort |
High baseline PLT count cohort |
|||
|---|---|---|---|---|
|
DOPTELET (60 mg) |
Placebo |
DOPTELET (40 mg) |
Placebo |
|
|
|
||||
|
Responder, n (%) |
51 (66.2) |
8 (19.5) |
39 (88.6) |
9 (37.5) |
|
95% CI |
(55.7, 76.8) |
(7.4, 31.6) |
(79.3, 98.0) |
(18.1, 56.9) |
|
95% CI of diff. |
46.7 (30.6, 62.8) |
|
51.1 (29.6, 72.6) |
|
|
>= 65 to |
||||
|
Responder, n (%) |
8 (66.7) |
2 (33.3) |
12 (85.7) |
4 (50.0) |
|
95% CI |
(40.0, 93.3) |
(0.0, 71.1) |
(67.4, 100.0) |
(15.4, 84.6) |
|
95% CI of diff. |
46.7 (-12.9, 79.5) |
|
35.7 (-3.5, 74.9) |
|
|
>= 75 Years old |
||||
|
Responder, n (%) |
0 |
1 (100) |
1 (100) |
0 |
|
95% CI |
(0.0, 0.0) |
(100.0, 100.0) |
(100.0, 100.0) |
(0.0, 0.0) |
Table 8. Summary of Proportion of Responders by Age Group -Trial 2
|
Age Group |
Low baseline PLT Count Cohort |
High Baseline PLT Count Cohort |
||
|---|---|---|---|---|
|
DOPTELET (60 mg) |
Placebo |
DOPTELET(40mg) |
Placebo |
|
|
|
||||
|
Responder, n (%) |
32 (71.1) |
13 (43.3) |
36 (83.7) |
8 (34.8) |
|
95% CI |
(57.9, 84.4) |
(25.6, 61.1) |
(72.7, 94.8) |
(15.3, 54.2) |
|
95% CI of diff. |
27.8 (5.6, 49.9) |
|
48.9 (26.6, 71.3) |
|
|
>= 65 to |
||||
|
Responder, n (%) |
12 (66.7) |
2 (18.2) |
12 (100) |
3 (42.9) |
|
95% CI |
(44.9, 88.4) |
(0.0, 41.0) |
(100.0, 100.0) |
(6.2, 79.5) |
|
95% CI of diff. |
48.5 (17.0, 80.0) |
|
57.1 (20.5, 93.8) |
|
|
>= 75 Years old |
||||
|
Responder, n (%) |
4 |
0 (100) |
3 (100) |
0 |
|
95% CI |
(20.5, 93.8) |
(0.0, 0.0) |
(100.0, 100.0) |
(0.0, 0.0) |
|
95% CI of diff. |
57.1 (20.5, 93.8) |
|
100.0 (100.0, 100) |
|
FDA Review
What are the possible side effects?
DOPTELET may cause serious side effects such as blood clots.
The most commonly reported side effects are fever, abdominal pain, nausea, headache, fatigue and swelling of the feet or hands.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions in the pooled clinical trials based on safety population defined as all patients who received at least 1 dose of trial drug and had at least 1 post-dose safety assessment.
Table 9. Adverse Reactions with a Frequency ≥3% in Patients treated with DOPTELET - Pooled Data Trial-1 and Trial-2|
|
Low Baseline Platelet Count Cohort |
High Baseline Platelet Count Cohort |
Combined Baseline |
|||
|---|---|---|---|---|---|---|
|
Adverse Reactionsa |
DOPTELET 60mg |
Placebo |
DOPTELET 40 mg |
Placebo |
Total |
Total |
|
Pyrexia |
11 |
9 |
8 |
9 |
10 |
9 |
|
Abdominal Pain |
6 |
7 |
7 |
6 |
7 |
6 |
|
Nausea |
6 |
8 |
7 |
6 |
7 |
7 |
|
Headache |
4 |
8 |
7 |
5 |
6 |
6 |
|
Fatigue |
4 |
4 |
3 |
2 |
4 |
3 |
|
Edema Peripheral |
3 |
2 |
4 |
2 |
3 |
2 |
aTreatment emergent adverse reactions are sorted in descending order by Total DOPTELET-treated patients (N=274)
DOPTELET Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: The occurrence of side effects among races was similar.
- Age: The occurrence of side effects was similar in patients younger and older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes adverse events in the pooled clinical trials by subgroups (safety population).
Table 10. Subgroup Analysis of Treatment Emergent Adverse Events(TEAE) in Pooled Trials|
Demographic Parameters |
DOPTELET |
Placebo |
|---|---|---|
|
Patients with Any TEAE (n, %) |
148/274 (54%) |
86/156 (51%) |
|
Sex (n, %) |
||
|
Men |
90/183 (49.2) |
51/98 (52) |
|
Women |
58/91 (63.7) |
35/58 (60.3) |
|
Race (n, %) |
||
|
White |
79/159 (49.7) |
46/98 (46.9) |
|
Asian |
55/93 (59.1) |
33/49 (67.3) |
|
Black or African American |
6/9 (66.7) |
2/2 (100) |
|
All Other |
8/13 (61.5) |
5/7 (71.4) |
|
Age Group (n, %) |
||
|
102/206 (53.7) |
65/117 (55.6) |
|
|
≥ 65 years |
46/68 (67.7) |
21/39 (53.8) |
Clinical Trial data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved DOPTELET based on evidence from two clinical trials, Trial 1 (NCT01972529) and Trial 2 (01976104), of 435 patients with low platelet counts because of chronic liver disease.
Trials were conducted at the sites located in the United States, Canada, Europe, Asia, Australia, Brazil and Argentina.
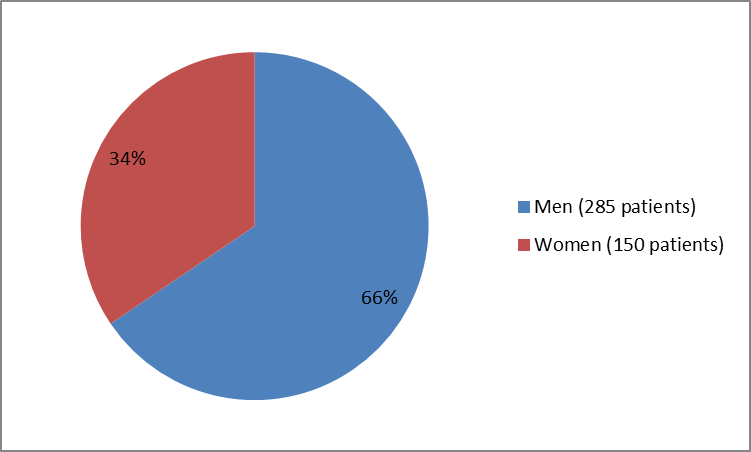
Figure 1 summarizes how many men and women were in the clinical trials.
Figure 1. Baseline Demographics by Sex
FDA Review
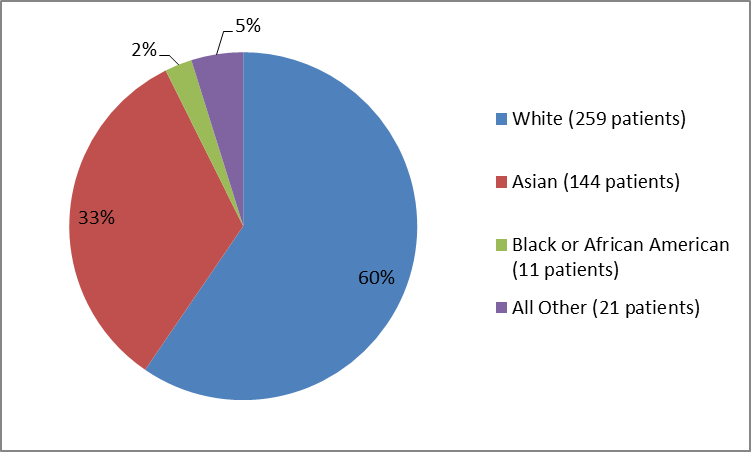
Figure 2 summarizes the percentage of patients by race in the clinical trials.
Figure 2 Baseline Demographics by Race
Table 1. Baseline Demographics by Race
|
Race |
Number of Patients |
Percentage |
|---|---|---|
|
White |
259 |
60 |
|
Asian |
144 |
33 |
|
Black or African American |
11 |
2 |
|
Other |
14 |
3 |
|
Missing |
7 |
2 |
FDA Review
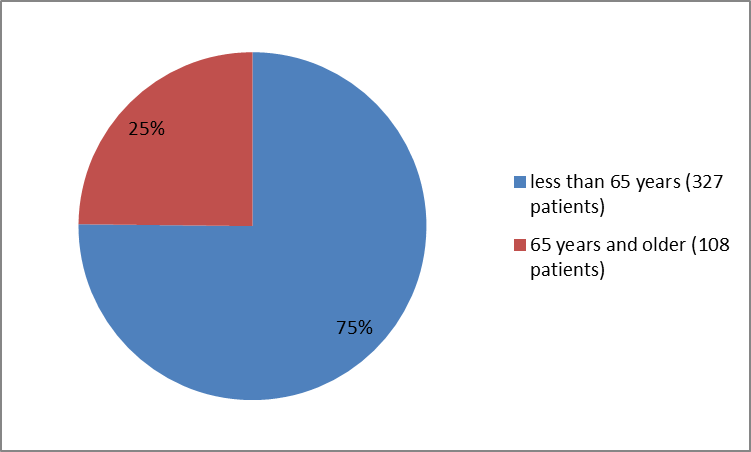
Figure 3 summarizes the percentage of patients by age in the clinical trials.
Figure 3. Baseline Demographics by AgeFDA Review
The table below summarizes demographics of patients that participated in both trials.
Table 11. Patient Demographics in Trials 1 and 2 Combined (Efficacy Population)
|
Demographic Parameters |
DOPTELET |
Placebo |
TOTAL |
|---|---|---|---|
|
Sex (n, %) |
|||
|
Men |
185 (66.8) |
100 (63.3) |
285 (65.5) |
|
Women |
92 (33.2) |
58 (36.7) |
150 (34.5) |
|
Race (n, %) |
|||
|
White |
161 (58.1) |
98 (62.0) |
259 (59.5) |
|
Black or African American |
9 (3.2) |
2 (1.3) |
11 (2.5) |
|
Asian |
93 (33.6) |
51 (32.3) |
144 (33.1) |
|
American Indian or Alaska Native |
0 |
0 |
0 |
|
Native Hawaiian or Other Pacific Islander |
0 |
0 |
0 |
|
Other |
8 (2.9) |
6 (3.8) |
14 (3.2) |
|
Missing |
6 (2.2) |
1 (0.6) |
7 (1.6) |
|
Age |
|||
|
Mean years (SD) |
57.3 (11.19) |
57.1(11.15) |
57.2 (11.16) |
|
Median (years) |
58 |
58 |
58 |
|
Min, max (years) |
19,85 |
25,81 |
19,85 |
|
Age Group (n,%) |
|||
|
209 75.5) |
118 (74.7) |
327 (75.2) |
|
|
≥ 65 years |
68 (24.5) |
40 (25.4) |
108 (24.8) |
|
Ethnicity (n, %) |
|||
|
Hispanic or Latino |
43 (16) |
32 (20) |
75 (17.7) |
|
Not Hispanic or Latino |
226 (84.0) |
122 (79.2) |
348 (82.3) |
|
Missing |
8 |
4 |
12 |
|
Region (n, %) |
|||
|
United States |
56 (20.2) |
28 (17.7) |
84 (19.3) |
|
Canada |
2 (0.7) |
3 (1.9) |
5 (1.1) |
|
Europe |
91 (32.9) |
54 (32.4) |
145 (33.3) |
|
East Asia |
89 (32.1) |
50 (31.6) |
139 (32.0) |
|
Rest of World |
39 (14.1) |
23 (14.6) |
62 (14.3) |
FDA Review
How were the trials designed?
The trials enrolled adult patients with low platelet counts who were scheduled to have a procedure that could lead to increased bleeding. Low platelet counts were consequence of chronic liver disease.
All patients were divided in two groups based on their baseline platelet counts: low platelet count and high platelet count. After that, all patients were randomly assigned to receive either DOPTELET or placebo tablets once a day for 5 consecutive days. Patients with low platelet count received DOPELET 60 mg and patients with higher platelet count received DOPELET 40 mg daily.
The benefit of DOPTELET was assessed based on the percentage of patients who did not require a platelet transfusion or any other rescue intervention for bleeding up to 7 days following the procedure.
How were the trials designed?
FDA approved DOPTELET based on data from two identical, randomized, placebo-controlled, multi-center trials in patients with chronic liver disease who were scheduled to undergo a procedure. Patients were assigned to the Low Baseline Platelet Count Cohort (˂40 x 109L) or the High Baseline Platelet Count Cohort (≥40 to ˂50 x 109 L) based on their platelet count at baseline. Patients were then randomized in a 2:1 ratio to either DOPTELET (60 mg or 40 mg,) or placebo once daily for 5 days.
In both trials, the primary endpoint was the proportion of patients who did not require a platelet transfusion or any rescue procedure for bleeding after randomization and up to 7 days following an elective procedure. Secondary efficacy outcomes were the proportion of patients who achieved platelet counts of >50 x109/L on the day of procedure and the change in platelet count from baseline to procedure day.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION