FDA's HSP/BIMO Initiative Accomplishments: Update May 2012
The Food and Drug Administration (FDA) has continued the Human Subject Protection (HSP)/Bioresearch Monitoring (BIMO) Initiative, , intended to modernize and strengthen the agency’s oversight and protection of subjects in clinical trials and the integrity of resulting data. The HSP/BIMO Initiative encompasses all FDA-regulated clinical trials, that is, those related to human drugs and biological drug products, devices, foods, and veterinary medicine. The overarching goals of the agency's BIMO program are to protect the rights, safety, and welfare of subjects involved in FDA-regulated clinical trials; to determine the accuracy and reliability of clinical trial data submitted to FDA in support of research or marketing applications; and to assess compliance with FDA's regulations governing the conduct of clinical trials, including those for informed consent and ethical review.
Below are significant accomplishments and initiatives since the last report.
- New Regulations
- New Guidance
- Draft Guidance
- Improvements to FDA's Internal Procedures
- Clinical Trial Transformation Initiative (CTTI)
- International Harmonization, Capacity-Building, and Outreach Activities
- Guidance in Development
- Modernizing FDA Regulations
- BIMO Inspections for Fiscal Year 2011
- Footnotes
- Investigational New Drug Safety Reporting Requirements for Human Drug and Biological Products and Safety Reporting Requirements for Bioavailability and Bioequivalence Studies in Humans – Final Rule.(4) This revision of the safety reporting requirements for human drug and biological products subject to an investigational new drug application (IND) codifies FDA’s expectations for timely review, evaluation, and submission of relevant and useful safety information and implements internationally harmonized definitions and reporting standards. The revisions will improve the utility of IND safety reports, reduce the number of reports that do not contribute in a meaningful way to the developing safety profile of the drug, expedite FDA’s review of critical safety information, subject bioavailability and bioequivalence studies to safety reporting requirements, and enable the agency to better protect human subjects and promote public health. A companion draft guidance was developed and published simultaneously. (September 2010)
- Informed Consent Elements – Final Rule.(5) The Food and Drug Administration Amendments Act of 2007 (FDAAA) requires that FDA update its informed consent regulations to require that the informed consent documents and processes for certain clinical investigations include a statement that clinical trial information for such investigations has been or will be submitted for inclusion in the National Institutes of Health/National Library of Medicine clinical trial registry databank. This final rule addresses this new requirement for informed consent. (January 2011)
- Disqualification of a Clinical Investigator – Final Rule.(6) FDA amended the regulations to extend clinical investigator disqualification to include all FDA-regulated investigational products. Under the final rule, an investigator determined by FDA to be ineligible to receive a particular investigational product is also deemed to be ineligible to receive any FDA-regulated investigational product. This rulemaking responds to recommendations by the Government Accountability Office and harmonizes existing investigator disqualification regulations. (April 2012)
- Good Laboratory Practice for Non-Clinical Laboratory Studies – Advanced Notice of Proposed Rulemaking (ANPRM).(7) Nonclinical studies have changed markedly since issuance of the good laboratory practice (GLP) regulation (21 CFR part 58) in 1978. In recognition of this change, FDA sought comment on whether to amend the regulation. The ANPRM summarized FDA’s considerations for modernization of the GLP regulation. Based on FDA’s review of the 1978 rule and preliminary stakeholder input, FDA believes that requiring nonclinical facilities/laboratories to follow a GLP quality system will help ensure the integrity of data in nonclinical studies. Although many of the requirements of the existing regulation are consistent with a GLP quality system, FDA proposed modifications to incorporate all basic elements needed for a GLP quality system consistent with internationally recognized quality systems. FDA believes that implementation of a GLP quality system would institute a risk-based approach, reduce regulatory burden, and encourage science-based technology. (December 2010)
- Exception from Informed Consent Requirements for Emergency Research – Final Guidance.(8) This guidance provides advice to Institutional Review Boards (IRBs), clinical investigators and sponsors in the development, conduct, and oversight of investigations to determine the safety and effectiveness of FDA-regulated products in emergency settings when an exception from the informed consent requirements is requested under 21 CFR 50.24. In particular, the guidance clarifies FDA’s expectations related to planning and conducting community consultation and public disclosure activities, and establishing informed consent procedures to be used when feasible. (March 2011)
- Questions and Answers on Informed Consent Elements, 21 CFR 50.25(c) – Final Guidance.(9) This guidance was developed to help sponsors understand the new informed consent regulation. The new regulation requires informed consent documents and processes for certain clinical investigations to include a statement that clinical trial information for such investigations has been or will be submitted for inclusion in the National Institutes of Health/National Library of Medicine clinical trial registry databank. (February 2012)
- Institutional Review Board (IRB) Continuing Review After Clinical Investigation Approval – Final Guidance.(10) FDA developed this guidance to provide more detail on the criteria, process, and frequency of continuing review and thereby assist IRBs, sponsors, and clinical investigators in protecting the rights and welfare of study subjects. (February 2012)
- FDA Acceptance of Foreign Clinical Studies Not Conducted Under an IND – Frequently Asked Questions – Final Guidance.(11) This guidance clarifies how sponsors and applicants may demonstrate compliance with the requirements of 21 CFR 312.120. It provides recommendations for the submission of information, whether in an IND or application for marketing approval for a drug or biological product, to demonstrate that a non-IND foreign clinical study was conducted in accordance with good clinical practice (GCP). (March 2012)
- In Vitro Diagnostic (IVD) Device Studies - Frequently Asked Questions.(12) This guidance is intended to assist the manufacturer, sponsor, applicant, investigator and the IVD device industry in the development of IVD studies, particularly those that are exempt from most of the requirements of the Investigational Device Exemption (IDE) regulation and to provide the regulatory framework pertaining to the development phase of IVD devices. (June 2010; this item was inadvertently omitted from the previous HSP/BIMO Initiative Update)
- Safety Reporting Requirements for INDs and BA/BE Studies.(13) This document provides guidance to sponsors and investigators on safety reporting requirements for human drug and biological products that are being investigated under an IND and for drugs that are the subjects of bioavailability (BA) and bioequivalence (BE) studies that are exempt from the IND requirements. This draft guidance contains definitions used for safety reporting, makes recommendations on when and how to submit a safety report, and provides advice on other safety reporting issues that have generated questions from sponsors and investigators. (September 2010)
- Investigational New Drug Applications (INDs) – Determining Whether Human Research Studies Can Be Conducted Without an IND.(14) This guidance is intended to assist clinical investigators, sponsors, and sponsor-investigators in determining whether human research studies must be conducted under an IND. With certain exceptions, clinical investigations in which a drug is administered to human subjects must be conducted under an IND as required in 21 CFR part 312. This draft guidance describes when an IND is required, specific situations in which an IND is not required, and a range of issues that, in FDA’s experience, have been sources of confusion or misperceptions about the application of the IND regulations. (October 2010)
- Electronic Source Documentation in Clinical Investigations.(15) This document provides guidance to sponsors, contract research organizations (CROs), data management centers, and clinical investigators on capturing, using, and archiving electronic data in FDA-regulated clinical investigations. This draft guidance is intended to ensure the reliability, quality, integrity, and traceability of electronic source data and source records maintained at the site for FDA inspection. (December 2010)
- Financial Disclosure by Clinical Investigators.(16) This updated guidance responds to recommendations aimed at strengthening FDA’s oversight and review of clinical investigators’ financial disclosures. Specifically, the guidance describes: (1) the sponsor's responsibility to collect the information prior to an investigator participating in a study and ensure that all required forms and attachments are submitted in marketing applications; (2) what is meant by “due diligence” in obtaining financial disclosures from investigators; and (3) how FDA will review financial disclosure information. The draft guidance seeks comment on the circumstances under which FDA should consider public release of financial disclosure information related to an approved marketing application. (May 2011)
- Oversight of Clinical Investigations – A Risk-Based Approach to Monitoring.(18) This guidance is intended to assist sponsors of clinical investigations in developing risk-based monitoring strategies and plans for investigational studies of medical products, including human drug and biological products, medical devices, and combinations thereof. This draft guidance describes strategies for monitoring activities that focus on critical study parameters and rely on a combination of monitoring methods to oversee a study effectively, such as greater use of centralized monitoring, where appropriate. (August 2011)
- Design Considerations for Pivotal Clinical Investigations for Medical Devices.(19) This guidance is intended to assist those involved in designing clinical studies intended to support premarket submissions for medical devices and FDA staff who review those submissions. This draft guidance describes different study design principles relevant to the development of medical device clinical studies that may be used to fulfill premarket clinical data requirements. (August 2011)
- Guidance on Exculpatory Language in Informed Consent.(20) This document provides guidance on the regulatory prohibition on the inclusion of exculpatory language in informed consent documents. This document was jointly issued with the Office of Human Research Protections (OHRP) and includes examples of language that OHRP and FDA consider acceptable as well as examples of language that both agencies would consider exculpatory. (August 2011)
- FDA Decisions for Investigational Device Exemption (IDE) Clinical Investigations.(21) FDA approval of an IDE submission allows the initiation of a clinical investigation of a significant risk device. This draft guidance is intended to provide clarification regarding the regulatory implications of the decisions that FDA may render based on review of an IDE and to provide a general explanation of the reasons for those decisions. (November 2011)
- Investigational Device Exemptions (IDE) for Early Feasibility Medical Device Clinical Studies, Including Certain First in Human (FIH) Studies.(23) This document is intended to provide guidance on the development and review of IDE applications for early feasibility studies of significant risk devices. Early feasibility studies allow for early clinical evaluation of devices to provide proof of principle and initial clinical safety data. These studies may be appropriate early in device development when clinical experience is necessary because nonclinical testing methods are not available or adequate to provide the information needed to advance the developmental process. However, as with all clinical studies, initiation of an early feasibility study must be justified by an appropriate risk-benefit analysis and adequate human subject protection measures. (November 2011)
- Determining the Extent of Safety Data Collection Needed in Late Stage Premarket and Postapproval Clinical Investigations.(24) This guidance is intended to assist sponsors of clinical trials of investigational drug and biological products in determining the amount and types of safety data to collect during late stage premarket and postmarket clinical investigations. The draft guidance emphasizes a selective and better targeted approach to safety data collection during late stage development or during the postmarket stage based on what is already known about a medical product’s safety profile. (February 2012)
Improvements to FDA’s Procedures
- Privacy Act System of Records – Notice.(25) FDA is altering an existing system of records, the “Bioresearch Monitoring Information System, HHS/FDA,” that provides controls to ensure that clinical investigators meet the requirements of the relevant statutes and regulations governing FDA-regulated products. The system also supports the effective performance of activities necessary for conducting FDA’s bioresearch monitoring program. The updates provide for new routine uses for disclosing certain relevant information to agencies, authorities, and organizations with responsibilities related to clinical investigations and/or clinical investigators; persons who require access to records to perform services for FDA; and individual research subjects. (January 2012)
- Warning Letter Initiative.(26) In 2009, FDA implemented a pilot program that establishes a 15 business day timeframe for the submission of post-inspection responses to FDA 483 observations. The pilot mandated that FDA would conduct a detailed review of any timely responses received before issuing a warning letter. If, after reviewing a firm’s timely response, FDA determined a warning letter was necessary, the warning letter would acknowledge receipt of the response and reply as to the apparent adequacy of the firm’s described corrective actions. The purpose of this program was to facilitate the timely issuance of warning letters, promote prompt correction of violations, and promote efficient use of agency resources. FDA is in the process of assessing the ongoing pilot to determine whether to make the program permanent.
- Prioritization of Clinical Trial Inspections. FDA’s Centers have developed new approaches for improving the process for selecting clinical investigators and other entities for inspection, both at the pre-approval stage and earlier in the product development process. For example, the Center for Devices and Radiological Health (CDRH) and the Center for Biologics Evaluation and Research (CBER) use early intervention programs whereby ongoing studies are identified for inspection using risk-based criteria (e.g., studies involving vulnerable patient populations, novel technologies, or investigational products, such as vaccines, that have a potentially large public health impact).
The Center for Drug Evaluation and Research (CDER) has continued to pilot a risk-based inspection model that permits more rapid identification of clinical sites identified for inspections related to New Drug Applications (NDAs). Thus far, CDER has utilized this new model for selecting sites for more than a dozen NDAs and anticipates expanding its use. The Center is also developing similar risk-based models to prioritize Institutional Review Board (IRB), clinical and analytical facilities (related to generic drug applications), and clinical trial sponsors and contract research organizations (CROs) for inspection, integrating information from applications, complaints, and other data sources.
- Compliance Program Guide Manual (CPGM) – Chapters on Sponsors, CROs, and Monitors (7348.810) and IRBs (7348.809).(27) Both chapters were revised to incorporate specific recommendations of the Office of the Inspector General (OIG) for improving communications among FDA staff before, during, and after an inspection and to more clearly define the thresholds for initiating regulatory actions against non-compliant firms.
- The Sponsor/CRO/ Monitor chapter also includes revised procedures to verify that sponsors are obtaining and maintaining required financial disclosures from investigators, and updating the disclosures (if appropriate) within one year of completion of the covered trial. (March 2011)
- The IRB chapter also incorporates changes in the IRB regulations since 1997, when this CPGM chapter was last revised (e.g., pediatric studies, studies of emergency treatments involving an exception from informed consent). (November 2011)
Clinical Trial Transformation Initiative (CTTI)(31)
In 2007, FDA and Duke University founded a public-private partnership whose goal is to identify practices that, if broadly adopted, would improve the quality and efficiency of clinical trials. To achieve this goal, CTTI conducts projects to generate empirical information about how clinical research is currently conducted and to identify and test ways to improve quality and efficiency. CTTI is composed of representatives from government, industry, patient advocacy groups, professional societies, academia, and international regulators. In 2011, CTTI completed the work and issued final recommendations on their two initial projects – Effective and Efficient Monitoring and Improving SAE Reporting to IND Investigators.(32)
An overview of CTTI’s latest projects (33) appears below:
- Workshops on Quality by Design in Clinical Trials. The CTTI monitoring project identified quality risk management (QRM), specifically quality by design (QbD) from the pharmaceutical manufacturing sector, as a potential model that, with adaptations, could contribute to high data quality and integrity in clinical trials. Such an approach recognizes that different aspects of clinical trials may present a higher compliance risk and permits both clinical trial sponsors and the agency to focus efforts on those activities that present a greater risk to data integrity and human subjects protection. In a clinical trial setting, sponsors would prospectively identify critical risks and then tailor protocol design and delivery to mitigate those risks.
To implement a quality risk management approach, participants in the monitoring expert meeting (October 2010) agreed that all stakeholders in the clinical trial process should have a common understanding of QRM principles and their application in a clinical trial setting. To facilitate dialogue on adapting QRM models to clinical development, CTTI convened a workshop in August 2011 with representatives from a broad cross-section of the clinical trial enterprise—including regulators, government, sponsors, academicians, industry representatives, patient advocates, clinical investigators, and other interested parties. The August workshop was the first in a series planned by CTTI as a learning exchange to explore best practices in prospectively designing quality into the clinical development lifecycle.
- IND Safety Assessment and Communication Project
FDA’s recent change in regulation on reporting of serious, unexpected, suspected adverse reactions (21 CFR parts 312 and 320) calls for IND sponsors to evaluate the data collected across all studies in an IND. Many sponsors have such internal monitoring committees to perform these safety assessments, but their composition and practices have not been well described. It is critical to understand these practices before one considers recommendations for future approaches that will support the intent of the new FDA rule on IND safety reporting.
The goal of this CTTI project is to promote responsible oversight of safety for pre-market products consistent with the intent of FDA’s new IND safety rule. The IND Safety Assessment and Communication Project seeks a deeper understanding of a sponsor’s current program-level, pre-market practices related to potential safety signals.
- Improving the Public Interface for Use of Aggregate Data in ClinicalTrials.gov
The ClinicalTrials.gov dataset is publicly available and can be downloaded directly from the website. Although the dataset has been optimized to access information about individual clinical trials, it remains difficult for most users to access aggregate information about clinical trials. CTTI is establishing and maintaining, via quarterly updates, a publicly accessible, user-friendly analysis dataset(34) of the ClinicalTrials.gov content, accompanied by a data dictionary. This will facilitate the use of aggregate data in ClinicalTrials.gov to evaluate the state of the clinical trials system over time.
- Site Metrics for Study Start-up
The goal of the site metrics project is to identify core data elements that should be collected by all clinical trial sites to enable measurement and improvement of important timeframes for study start-up. The team is analyzing timelines for start-up of phase III multicenter studies from several existing datasets. A pilot study will be initiated to prospectively collect metrics for start-up activities on a sample of phase III multicenter clinical trials. The team will evaluate potential mechanisms for continuous public posting of study start-up metrics.
- Use of Central IRBs for Multicenter Clinical Trials
The goal of this project is to identify potential solutions to address barriers to the adoption of central IRBs for multicenter clinical trials. The project team plans to solicit input about current perceptions of such barriers and proposed solutions. A strategy to address the identified barriers will then be developed.
Recent CTTI collaborations include:(35)
- Clinical Investigator Training Course. FDA and CTTI have co-sponsored 3 training courses for clinical investigators on scientific, ethical, and regulatory aspects of clinical trials from 2009 to 2011. To date, over 500 clinical investigators have participated in this training program.
- Standards for Collecting Information about Cardiovascular Events. This collaborative pilot project is intended to develop standard definitions and data collection methods for cardiovascular events in clinical trials (e.g., a standard Case Report Form for cardiovascular endpoint events). This effort may improve uniformity of data collection and analysis of results, and thereby allow better identification of safety signals and trends during the development of new biologics, devices, and drugs. In 2010, the group convened expert meetings and developed a reference document with draft definitions for endpoint events in cardiovascular trials. The draft definitions were posted for public comment in November 2010 as the first step in the process of being reviewed through the Clinical Data Interchange Standards Consortium (CDISC) and Health Level 7 International (HL7) processes.
International Harmonization, Capacity-Building, and Outreach Activities
Increasing globalization of clinical trials presents challenges to both U.S. and foreign regulators. To address these challenges, FDA has sought to leverage its resources more efficiently by engaging in collaboration and outreach with international regulatory authorities. Specific examples of these activities are listed below.
FDA conducted numerous programs to train other countries’ governments and international health regulatory bodies on good clinical practice (GCP) or to assist them in establishing or improving their GCP inspectional capacity. These activities include:
- Basic GCP “Train the Trainers” inspection training for member regulators from the Southern African Development Community (Gabarone, Botswana, October 2010)
- Intermediate (Phase 2) GCP inspection training for China’s State Food and Drug Administration (Guangzhou, February/March 2011)
- Intermediate (Phase 2) GCP inspection training for Russia’s Roszdravnadzor (Federal Service on Surveillance in Health and Social Development of the Russian Federation, Moscow, June/July 2011)
- Intermediate (Phase 2) GCP inspection training for regulators from the Southern African Development Community (Pretoria, South Africa, September 2011)
- Providing speakers and discussants to international stakeholder groups, including the Drug Information Association (DIA) Euromeeting (Geneva, March 2011)
- NAFTA (North Atlantic Free Trade Association)-region representation to the 6th Congress of the Pan American Network on Drug Regulatory Harmonization (PANDRH) and its GCP Working Group (Brasilia, Brazil, July 2011)
FDA-EMA Good Clinical Practices (GCP) Initiative. FDA’s Center for Drug Evaluation and Research and the European Medicines Agency (EMA) completed their 18-month pilot GCP Initiative which began on September 1, 2009. Key objectives of the initiative have been met and both agencies view the pilot as being productive and successful. Since the pilot began, FDA and the EMA have shared inspectional information on dozens of applications, have collaborated on numerous joint and observational inspections, have participated in bilateral trainings, and have kept each other informed of GCP-related legislation, regulatory guidance, and related documents. These activities have facilitated improvements in the agencies’ inspection coverage and decision-making processes and have contributed greatly to each agency’s understanding of the other’s inspection procedures. A final report on the pilot initiative was published on July 18, 2011. , Some of the considerations listed in the report include:
- Carrying out more EMA/FDA inspections in order to identify the gaps in each agency’s inspection processes;
- Focusing on joint inspections of sponsors and CROs as well as triggered inspections;
- Strengthening training and understanding of each region’s inspection procedures; and
- Exploring the possibility of expanding the initiative to other areas such as bioequivalence (BE) and to the Center for Biologics Evaluation and Research.
The EMA and FDA are continuing with the initiative, incorporating lessons learned during the pilot, and exploring opportunities for expanding its scope.
ANSI/AAMI /ISO 14155: 2011 Clinical investigation of medical devices for human subjects – Good clinical practice. Formally recognized by the FDA, ISO 14155:2011 is a guide on how to design/plan, conduct, record and report data from medical device clinical trials. It describes and defines the duties of the study sponsor, clinical investigator and monitor, includes a detailed section on the ethical conduct and protection of human subjects, and provides detailed annexes specifying the content and format of essential clinical trial documents. FDA's Center for Devices and Radiological Health (CDRH) served on a committee composed of international regulatory agencies and organizations to develop this updated standard. This standard will help ensure that international medical device trials are conducted in accordance with good clinical practices, that the data and reported results from international trials are credible and accurate, and that the rights, safety, and well-being of trial subjects are protected. (March 2012)
- A Guide to Informed Consent – Draft Guidance. This guidance describes in detail basic and additional elements of informed consent and includes topics such as review of patient records, children as subjects, and subject participation in more than one study.
- Consideration When Transferring Clinical Investigation Oversight to Another IRB – Draft Guidance. This guidance discusses the regulatory responsibilities of clinical investigators, sponsors, and IRBs when oversight of a previously approved clinical investigation is transferred from one IRB to another IRB. The draft guidance also addresses questions that have been previously raised concerning procedures and processes that are required and/or recommended by FDA when such oversight is transferred.
- Additional Protections for Children Enrolled in Research (21 CFR part 50 subpart D) – Draft Guidance. This guidance is intended to assist sponsors, clinical investigators, and IRBs in the development, conduct, and oversight of pediatric research involving FDA-regulated products. The draft guidance will discuss different pathways for pediatric product development that are consistent with 21 CFR 50 subpart D, and the ethical and regulatory issues that need to be considered.
- Core Responsibilities of Institutional Review Boards – Draft Guidance. IRBs are responsible for the review and approval of human subject research in order to protect the rights, safety, and welfare of subjects. This draft guidance is being developed jointly with the Office of Human Research Protections (OHRP) and is intended to provide assistance to the research community in interpreting IRB core responsibilities as required by FDA and HHS regulations.
- Safety Reporting Requirements for INDs and BA/BE Studies – Small Entity Compliance Guide – Final Guidance. This guidance is intended to help small businesses understand and comply with FDA’s safety reporting regulations for human drug and biological products that are being investigated under an IND and for drugs that are the subjects of bioavailability (BA) and bioequivalence (BE) studies that are exempt from the IND requirements.
- Modernizing the Regulation of Clinical Trials and Approaches to Good Clinical Practice – Public Hearing. FDA is actively working to modernize the regulatory framework that governs clinical trials and approaches to good clinical practice (GCP). Clinical trials are a critical source of evidence to inform medical policy and practice, and effective regulatory oversight is needed to ensure that human subjects are protected and resulting clinical trial data are credible and accurate. FDA is aware of concerns within the clinical trial community that certain regulations and policies applicable to the conduct of clinical trials may result in inefficiencies or increased cost and may not facilitate the use of innovative methods and technological advances to improve clinical trial quality. On April 23, 2012, FDA held a public hearing40 to solicit public input from a broad group of interested stakeholders on the scope and direction of this effort.
- Additional Safeguards for Children in Clinical Investigations of FDA-Regulated Products – Final Rule. This rule finalizes the interim rule adopted in 2001 to bring FDA regulations into compliance with provisions of the Children’s Health Act of 2000 (the Children’s Health Act). The Children’s Health Act requires that all research involving children that is conducted, supported, or regulated by the Department of Health and Human Services (HHS) be in compliance with HHS regulations providing additional protections for children involved as subjects in research. FDA is taking this action both to comply with the congressional mandate and because of increased enrollment of children in clinical investigations as a result of ongoing pediatric initiatives.
- Reporting Information Regarding Falsification of Data – Final Rule.(41) FDA proposed to amend its regulations to require sponsors to report information indicating that any person has, or may have, engaged in the falsification of data in the course of reporting study results, or in the course of proposing, designing, performing, recording, supervising, or reviewing studies that involve human subjects or animal subjects conducted by or on behalf of a sponsor or relied on by a sponsor. FDA proposed this change because ambiguity in the current reporting scheme has caused confusion among sponsors. The final rule is intended to help ensure the validity of data that the FDA receives in support of applications and petitions for FDA product approvals and authorization of certain labeling claims and to protect research subjects.
- Acceptance of Data From Clinical Studies for Medical Devices – Notice of Proposed Rulemaking.(42) FDA is proposing to amend its regulations on acceptance of data from clinical studies conducted outside the United States in support of a premarket approval application or a humanitarian device exemption application for a medical device. The proposed rule would require that these studies be conducted in accordance with GCP. FDA proposes to define GCP as a standard for the design, conduct, performance, monitoring, auditing, recording, analysis, and reporting of clinical trials in a way that provides assurance that the data and reported results are credible and accurate, and that the rights, safety, and well-being of trial subjects are protected. GCP also would include review and approval by an independent ethics committee (IEC) before initiating a study, continuing IEC review of ongoing studies, and obtaining and documenting freely given informed consent of study subjects. FDA is also proposing to include requirements for the submission of clinical data in support of an investigational exemption application and a premarket notification submission. The proposed changes will require a statement regarding compliance with FDA regulations for studies conducted in the United States and compliance with GCP for studies conducted outside the United States. With the above described changes, the proposed rule is intended to update the standards for the acceptance of clinical studies and to help to continue to ensure the protection of human subjects and the quality and integrity of data obtained from these studies.
BIMO Inspection Metrics for FY 2011
Each year, FDA's field staff conduct on-site inspections of BIMO establishments, including sponsors, monitors, clinical investigators, IRBs, and laboratories that conduct nonclinical safety studies (including animal toxicity studies) to support FDA-regulated research. The agency performs these inspections to determine whether the inspected party's practices and procedures comply with applicable regulations. Summary information about FDA's inspectional activities for fiscal year 2011 (42) is presented below:
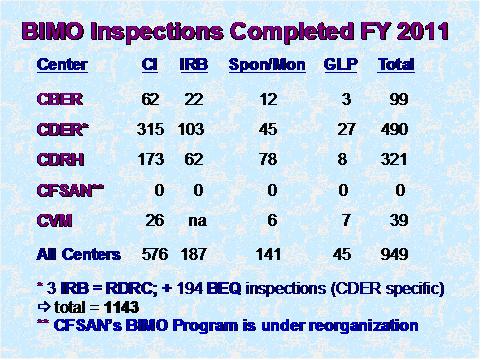
Figure 1
Figure 1 depicts the total number of inspections that were completed by the Office of Regulatory Affairs' field investigators as well as the numbers completed for each of the assigning Centers. (BEQ denotes bioequivalence studies.)
Figure 2
Figure 2 depicts the compliance classification of EIRs for inspections of IRBs that were classified by the assigning Center in FY 2011.
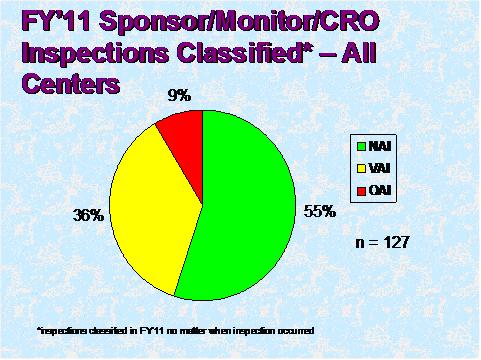
Figure 3
Figure 3 depicts the compliance classification of EIRs for inspections of sponsors that were classified by the assigning Center in FY 2011
Figure 4
Figure 4 depicts the compliance classification of EIRs for inspections of bioequivalence studies that were classified in FY 2011.
Figure 5
Figure 5 depicts the compliance classification of EIRs for inspections of international clinical investigators that were classified in FY 2011.
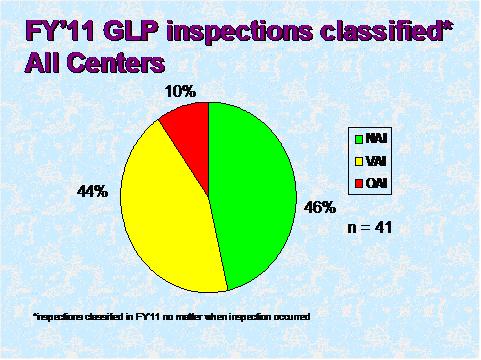
Figure 6
Figure 6 depicts the compliance classification of EIRs for inspections of GLP facilities that were classified in FY 2011.
1. The HSP/BIMO Council, which is overseeing this initiative, includes representatives from each of FDA's Centers, the Office of Regulatory Affairs (ORA), and the Office of the Commissioner (OC). These progress reports include HSP/BIMO accomplishments and initiatives from all FDA Centers and Offices.
2. Information on the HSP/BIMO Initiative and progress reports can be found at: HSP/BIMO Initiative. These progress reports include HSP/BIMO accomplishments and initiatives from all FDA Centers and Offices.
3. http://frwebgate.access.gpo.gov/cgi-bin/getdoc.cgi?dbname=2010_register&docid=fr29se10-3.pdf
4. Safety Reporting Requirements for INDs and BA/BE Studies
5. 21 CFR Part 50 - Informed Consent Elements Final Rule
6. 21 CFR Parts 16, 312, 511, and 812 - Disqualification of a Clinical Investigator, Final Rule
7. 21 CFR Part 58 - Good Laboratory Practice for Nonclinical Laboratory Studies - Advance notice of proposed rulemaking
8. Exception from Informed Consent Requirements for Emergency Research
9. Questions and Answers on Informed Consent Elements, 21 CFR § 50.25(c)
10. IRB Continuing Review After Clinical Investigation Approval
11. FDA Acceptance of Foreign Clinical Studies Not Conducted Under an IND: Frequently Asked Questions
12. In Vitro Diagnostic (IVD) Device Studies - Frequently Asked Questions
13. Safety Reporting Requirements for INDs (Investigational New Drug Applications) and BA/BE (Bioavailability/Bioequivalence) Studies
14. Investigational New Drug Applications (INDs) - Determining Whether Human Research Studies Can Be Conducted Without an IND
15. Electronic Source Data in Clinical Investigations
16. Financial Disclosure by Clinical Investigators
17. See the Office of the Inspector General (OIG), Department of Health and Human Services report, The Food and Drug Administration’s Oversight of Clinical Investigators’ Financial Information (OEI-05-07-00730)
18. Oversight of Clinical Investigations — A Risk-Based Approach to Monitoring
19. Design Considerations for Pivotal Clinical Investigations for Medical Devices
20. Exculpatory Language in Informed Consent
21. FDA Decisions for Investigational Device Exemption Clinical Investigations
22. Sponsor's Responsibilities For Significant Risk Device Investigations (Nov. 1995)
23. Investigational Device Exemptions (IDEs) for Early Feasibility Medical Device Clinical Studies, Including Certain First in Human (FIH) Studies
24. Determining the Extent of Safety Data Collection Needed in Late Stage Premarket and Postapproval Clinical Investigations
25. Privacy Act of 1974; Report of an Altered System of Records, Including Addition or Routine Uses to an Existing System of Records; Bioresearch Monitoring Information System, Federal Register January 9, 2012
26. Review of Post-Inspection Responses, Notice. Federal Register August 11, 2009
27. Bioresearch Monitoring Program (BIMO). FDA’s CPGM provides instructions to FDA personnel for conducting inspections to evaluate industry compliance with the Federal Food, Drug, and Cosmetic Act and other laws administered by FDA.
28. Compliance Program 7348.810 Bioresearch Monitoring, Sponsors, Contract Research Organizations, and Monitors
29. Compliance Program 7348.809, Bioresearch Monitoring, Institutional Review Boards
30. See the OIG report, The Food and Drug Administration's Oversight of Clinical Trials (OEI-01-06-00160).
31. Clinical Trials Transformation Initiative (CTTI)
32. For more details on these initial projects and CTTI’s final recommendations, see the following links:
CTTI Recommendations on Effective and Efficient Monitoring as a Component of Quality Assurance in the Conduct of Clinical Trials;
33. CTTI Projects
35. Clinical Trials Transformation Initiative (CTTI)
36. FDA News Release: FDA, European Medicines Agency Launch Good Clinical Practices Initiative
37. http://www.fda.gov/downloads/InternationalPrograms/FDABeyondOurBorders
ForeignOffices/EuropeanUnion/EuropeanUnion/EuropeanCommission/UCM266259.pdf
38. For a complete list of frequently asked questions regarding the EMA/FDA GCP initiative, please see Questions and answers EMA-FDA GCP initiative.
39. http://www.gpo.gov/fdsys/pkg/FR-2012-03-16/pdf/2012-6389.pdf
40. Modernizing the Regulation of Clinical Trials and Approaches to Good Clinical Practice; Public Hearing; Request for Comments
41. Reporting Information Regarding Falsification of Data, Proposed Rule, Federal Register February 19, 2010
42. http://www.gpo.gov/fdsys/pkg/FR-2012-02-13/pdf/2012-1647.pdf
43. Metrics for FY 2010 and previous years are available on FDA's website: BIMO Inspection Metrics.