FDA Taking New Steps to Better Inform Physicians about Biosimilars Through Education about these Potentially Cost-Saving Options
October 23, 2017
By: Scott Gottlieb, M.D., and Leah Christl, Ph.D.

FDA Commissioner Scott Gottlieb, MD
The cost of prescription drugs is an ongoing concern, however, a growing market for potentially lower-cost biological products called biosimilars can offer more competition and options for patients.
Biosimilars can potentially reduce costs for consumers by creating price competition for products that previously faced few market competitors. FDA wants to ensure that health care providers have the information they need when considering prescribing biosimilars when these products are available.
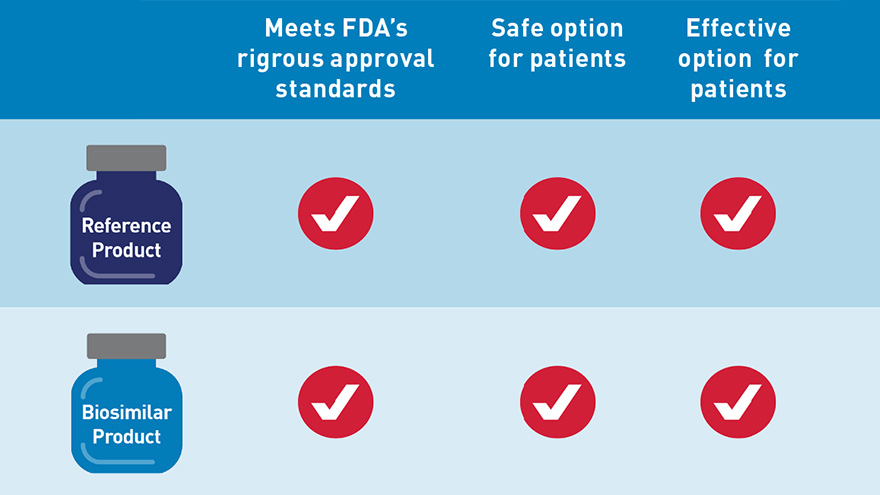
An FDA-approved biosimilar is highly similar to — and has no clinically meaningful differences in terms of safety, purity and potency (safety and effectiveness) from — an already FDA-approved biological product, called the “reference product.” In general, biological products are highly complex and are often used to treat patients with serious and life-threatening conditions.
FDA’s Center for Drug Evaluation and Research (CDER) has approved seven biosimilars to date. As more biosimilars are approved by FDA, we want health care providers to understand what these drugs are, and how they can help patients. So we are taking new steps to make sure providers are properly informed about biosimilars by launching an educational campaign today.
Leah Christl, Ph.D., Associate Director for Therapeutic Biologics in the Office of New Drugs, at FDA’s Center for Drug Evaluation and Research
We planned and researched extensively prior to developing the materials for this education and outreach effort. Through this process, we learned the specific areas that health care providers had questions about. These include questions about the data and information FDA reviews when it’s making decisions to approve a biosimilar. Based on that feedback, FDA has developed educational materials to help health care providers gain a better understanding of these important products and the approval process they undergo. This includes fact sheets and graphics for health care professionals, as well as materials for organizations to use in disseminating this information to their interested members. These resources:
- Provide the basic definitions of terms like: biological product, reference product, biosimilar, interchangeable; and other terms to facilitate understanding of the relationship between biosimilars and their reference products;
- Describe the rigorous standards any biosimilar must meet prior to approval and explain how the FDA approval pathway works for these products; and,
- Provide easily accessible information about the data and information FDA reviews to determine biosimilarity, and how to find more resources.
The new website also highlights information about an important reference for biosimilars known as the Purple Book. This reference can help prescribers and patients learn which biological reference products currently have one or more approved biosimilar or interchangeable product approved by FDA.
Next, FDA plans to embark on additional research with health care professionals to learn more about the types of information prescribers need to properly communicate with their patients about biosimilars. An increase in market competition, offered by a growing complement of biosimilars, may lead to meaningfully reduced costs for both patients and our healthcare system. As with the significant savings that we’ve seen through the introduction of generic drugs in the United States, biosimilars could also lead to substantial savings, thereby potentially improving access and promoting better public health outcomes. Understanding the rigorous process FDA uses to evaluate biosimilars can help prescribers and patients maximize the benefits from these products.
Scott Gottlieb, M.D., is Commissioner of the U.S. Food and Drug Administration
Leah Christl, Ph.D., is the Associate Director for Therapeutic Biologics in the Office of New Drugs, at FDA’s Center for Drug Evaluation and Research