Use of the Term Healthy on Food Labeling
Background | Proposed Rule
Proposed Criteria for Certain Food Groups and Sample Foods
Previous Actions | "Healthy" Symbol
Resources & Meetings
Nutrient Content Claim “Healthy"
Claims like “healthy” on food labels can provide information to consumers to help them identify healthier food choices at a quick glance. Foods must meet specific nutrient-related criteria to use the nutrient content claim “healthy.” The FDA has begun a public process to update the "healthy" claim for food labeling to be consistent with current nutrition science and federal dietary guidance.
Updating the “healthy” claim is one of the FDA’s nutrition initiatives, which seek to reduce the burden of chronic disease and advance health equity. The agency also remains committed to continuing to create a healthier food supply through its recently released guidance to reduce sodium in processed, packaged and prepared foods; to providing consumers with valuable and accessible nutrition and labeling information about the foods they eat; and to providing industry with recommendations on how to use and improve dietary guidance statements on food packaging.
U.S. Dietary Intakes & Recommendations
Proposed Rule
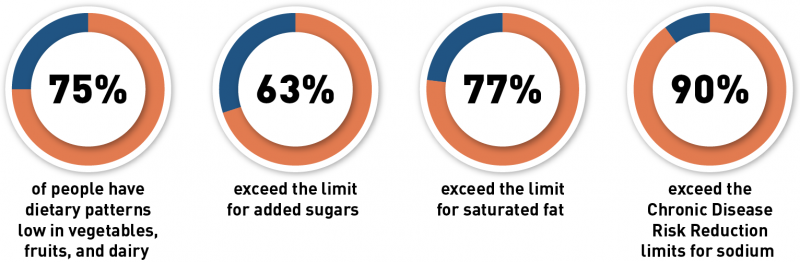
On September 28, 2022, the FDA issued a proposed rule to update the definition of the nutrient content claim “healthy,” which was set in 1994. The existing definition has limits for total fat, saturated fat, cholesterol and sodium and to qualify, foods must also provide at least 10% of the Daily Value (DV) for one or more of the following nutrients: vitamin A, vitamin C, calcium, iron, protein and fiber.
Use of the “healthy” claim is voluntary. The proposed changes to the definition of “healthy” are aligned with current nutrition science, federal dietary guidance, especially the Dietary Guidelines for Americans, 2020-2025, and the updated Nutrition Facts label. For example, current dietary guidelines focus on the importance of healthy dietary patterns and the food groups that comprise them, the type of fat in the diet rather than the total amount of fat consumed, and the amount of sodium and added sugars in the diet.
The FDA’s proposed framework for the updated definition of “healthy” focuses on ensuring that nutrient-dense foods that help consumers to build a diet consistent with current dietary recommendations can qualify to bear the claim. Specifically, to meet the proposed definition, a food product would need to contain a certain amount of food from at least one of the food groups or subgroups (e.g., fruit, vegetables, grains, dairy and protein foods) recommended by the 2020-2025 Dietary Guidelines for Americans. The specific limits for added sugars, saturated fat and sodium would be based on a percentage of the Daily Value for these nutrients. DVs are reference amounts of nutrients to consume or not to exceed each day. The proposed criteria for how much food from a particular food group is required (called food group equivalents) and the specific limits for the three nutrients vary for individual food products, mixed products (which contain more than one food group), main dishes and meals, and are based on a Reference Amount Customarily Consumed, which is the basis for determining a serving size. Under the proposed definition, raw whole fruits and vegetables would automatically qualify for the “healthy” claim because of their nutrient profile and positive contribution to an overall healthy diet.
Products that Could Qualify for “Healthy under the Proposed Rule
Proposed Criteria for Certain Food Groups and Sample Foods
Per Reference Amount Customarily Consumed
oz =ounce
g = grams
mg = milligrams
DV = Daily Value
| Food Groups | Food Group Equivalent Minimum |
Added Sugar Limit | Sodium Limit | Saturated Fat Limit |
|---|---|---|---|---|
| Grains | 3/4 oz whole-grain equivalent |
5% DV (2.5 g) |
10% DV (230 mg) | 5% DV (1 g) |
| Dairy | 3/4 cup equivalent | 5% DV (2.5 g) | 10% DV (230 mg) |
10% DV (2 g) |
| Vegetable | 1/2 cup equivalent | 0% DV (0 g) |
10% DV (230 mg) |
5% DV (1 g) |
| Fruit product | 1/2 cup equivalent |
0% DV (0 g) |
10% DV (230 mg) | 5% DV (1 g) |
| Proteins | Food Group Equivalent Minimum |
Added Sugar Limit | Sodium Limit | Saturated Fat Limit |
|---|---|---|---|---|
| Game meat |
1 ½ oz equivalent |
0% DV |
10% DV |
10% DV |
| Seafood |
1 oz equivalent |
0% DV |
10% DV |
10% DV |
| Egg |
1 egg |
0% DV |
10% DV |
10% DV |
| Beans, peas, and soy products |
1 oz equivalent |
0% DV |
10% DV |
5% DV |
| Nuts and seeds | 1 oz equivalent | 0% DV | 10% DV | 5% DV* |
|
* Excluding saturated fat derived from nuts and seeds |
||||
| Oils | Food Group Equivalent Minimum |
Added Sugar Limit | Sodium Limit | Saturated Fat Limit |
|---|---|---|---|---|
|
100% Oil |
N/A |
0% DV |
0% DV |
20% of total fat |
|
Oil-based |
N/A |
0% DV | 5% DV |
20% of total fat |
| Oil-based Dressing* |
N/A | 2% DV | 5% DV | 20% of total fat |
|
* Must contain at least 30% oil and saturated fat level of the oil must be ≤ 20 percent of total fat |
||||
|
Sample Foods |
Individual food |
Mixed product |
Meal |
|---|---|---|---|
|
Amount of food groups required |
6-oz yogurt (1 food group equivalent)* |
1/8 cup dried fruit and 1/4 oz nuts (At least 1/2 food group equivalent each from 2 different food groups) |
1 oz salmon, 1/2 cup green beans, 3/4 oz brown rice (At least 1 food group equivalent each from 3 different food groups) |
|
Nutrients to Limit (no more than)** |
2 g saturated fat |
1 g saturated fat*** |
4 g saturated fat |
|
* A food group equivalent is the amount of a food group required ** Amounts based on percentage of the Daily Value for that nutrient *** Saturated fat from nuts/seeds does not contribute to limit |
|||
Under the proposed definition, raw whole fruits and vegetables would automatically qualify for the “healthy” claim because of their nutrient profile and positive contribution to an overall healthy diet. Examples of foods currently ineligible to bear the “healthy” claim based on the existing regulatory definition, but that would qualify under the proposed definition are water, avocados, nuts and seeds, higher fat fish, such as salmon, and certain oils. Products that currently qualify for “healthy” that would not under the proposed definition include white bread, highly sweetened yogurt and highly sweetened cereal.
Previous Actions
During the FDA’s consideration of how to update the "healthy” claim, in September 2016, the FDA issued a guidance that states the agency’s intent to exercise enforcement discretion for products labeled with a “healthy” claim that meet certain nutrient requirements.
Also in September 2016, the FDA issued a request for information and comments related to the use of the term “healthy” in food labeling.
On March 9, 2017, the FDA held a public meeting to discuss use of the term “healthy” in food labeling.
“Healthy” Symbol
On a separate but related track, the FDA has begun to conduct research on a symbol that industry can voluntarily use to label food products that meet the proposed “healthy” definition. Symbols may be particularly helpful for those with lower nutrition knowledge to identify foods that can be the foundation of a healthy eating pattern. The FDA has issued two procedural notices on the preliminary quantitative consumer research it plans to conduct on voluntary symbols that could be used in the future to convey the nutrient content claim “healthy.” The first notice was issued in May 2021 and the second notice was issued in March 2022. Federal agencies are required to publish notice in the Federal Register on each proposed information collection and to give the public the opportunity to comment.
Resources & Meetings
- Webinar on the Proposed Changes to the Definition of “Healthy” (October 21, 2022)