Drug Trials Snapshots: GIAPREZA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the GIAPREZA Prescribing Information for complete information.
GIAPREZA (angiotensin II)
JEE-ah-prez-ah
La Jolla Pharmaceutical Company
Approval date: December 21, 2018
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
GIAPREZA is a drug used to increase dangerously low blood pressure in adults with certain types of shock.
Shock is a life-threatening condition in which blood pressure drops so low that the brain, kidneys, and other vital organs can't receive enough blood flow to function properly.
How is this drug used?
GIAPREZA is given by a health care professional directly into the bloodstream through a needle in the vein. This is known as an intravenous, or IV infusion. The amount of drug is based on patient’s blood pressure.
What are the benefits of this drug?
GIAPREZA increases blood pressure in patients with shock who could not reach adequate blood pressure on standard treatments used to raise blood pressure.
What are the benefits of this drug (results of trials used to assess efficacy)?
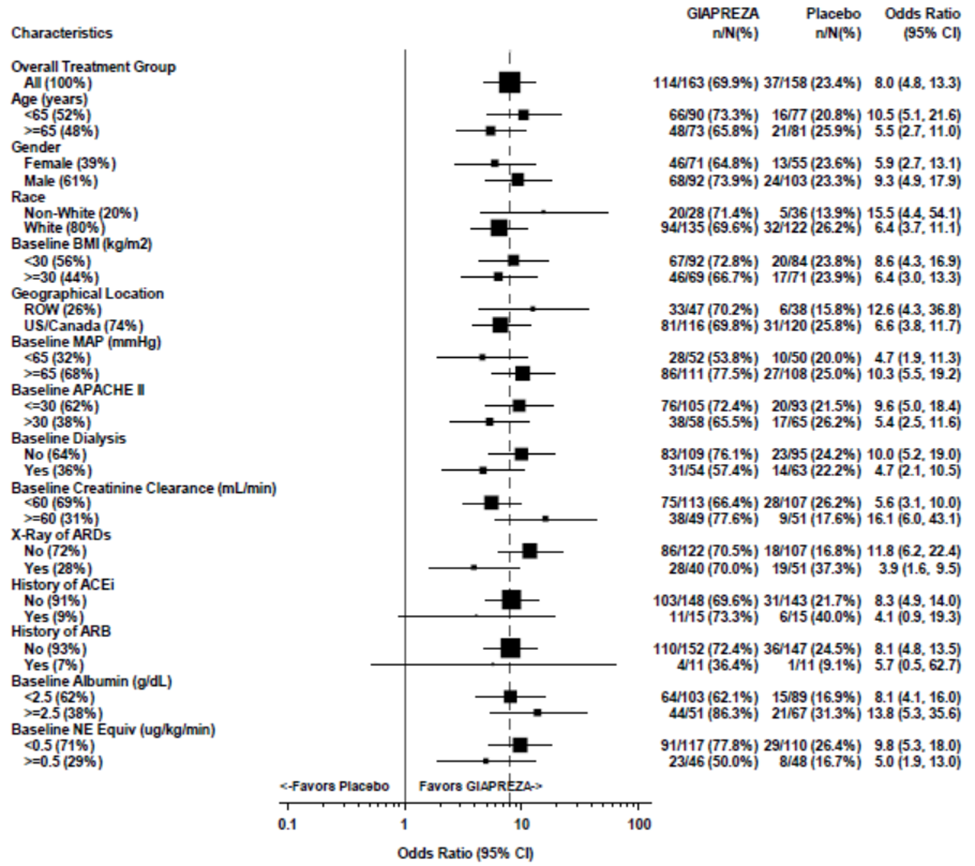
The primary endpoint defined as the percentage of patients who achieved either a mean arterial pressure (MAP) ≥ 75 mm Hg or a ≥ 10 mmHg increase in MAP without an increase in baseline vasopressor therapy at 3 hours was achieved by 70% of patients randomized to GIAPREZA compared to 23% of placebo subjects; p
Figure 4. Primary Endpoint – Overall Result and Results in Selected Subgroups
GIAPREZA Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: GIAPREZA worked similarly in men and women.
- Race: GIAPREZA worked similarly in White and non-White patients. The number of patients in races other than White was limited, therefore differences in response among individual non-White races could not be determined.
- Age: GIAPREZA worked similarly in patients above and below age 65.
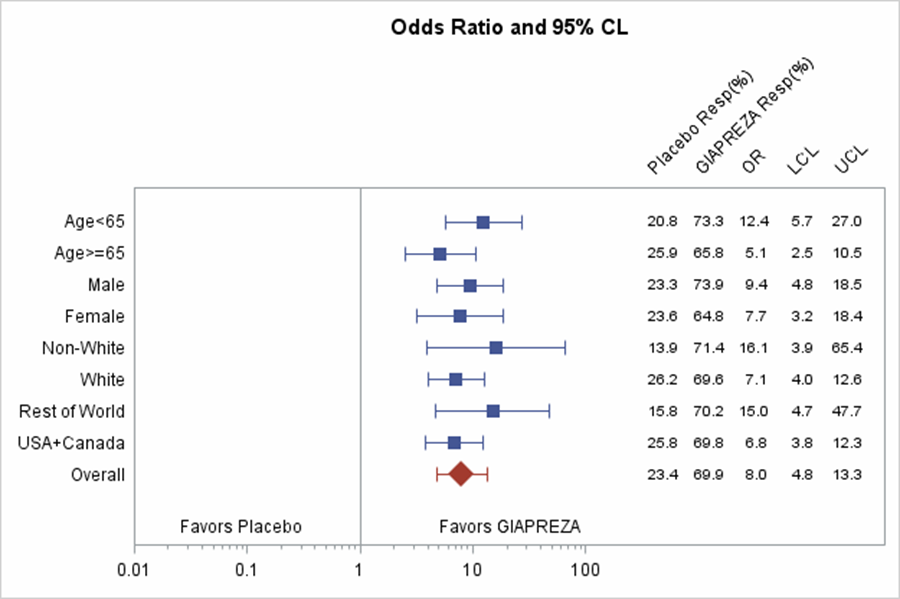
Were there any differences in how well the drug worked in clinical trials among sex, race and age groups?
Subgroup differences in MAP by age, sex and race are presented in the figure below.
Figure 5. Primary Endpoint and Selected Subgroup Analyses
FDA Statistical review
What are the possible side effects?
GIAPREZA may increase the chance of dangerous blood clotting in veins and arteries
What are the possible side effects (results of trials used to assess safety)?
Below is the summary of the most common adverse reactions observed in the trial.
Table 2: Adverse Reactions Occurring in ≥ 4% of Patients Treated with GIAPREZA and ≥ 1.5% More Often than in Placebo-treated Patients
Adverse Event | GIAPREZA | Placebo |
|---|---|---|
Thromboembolic eventsa | 21 (12.9%) | 8 (5.1%) |
Deep vein thrombosis | 7 (4.3%) | 0 (0.0%) |
Thrombocytopenia | 16 (9.8%) | 11 (7.0%) |
Tachycardia | 14 (8.6%) | 9 (5.7%) |
Fungal infection | 10 (6.1%) | 2 (1.3%) |
Delirium | 9 (5.5%) | 1 (0.6%) |
Acidosis | 9 (5.5%) | 1 (0.6%) |
Hyperglycemia | 7 (4.3%) | 4 (2.5%) |
Peripheral ischemia | 7 (4.3%) | 4 (2.5%) |
a Including arterial and venous thrombotic events
GIAPREZA Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: The occurrence of side effects was similar in White and non-White patients. The number of patients in each non-White race was small, therefore differences in response among individual non-White races could not be determined.
- Age: The occurrence of side effects was similar in patients above and below age 65.
Were there any differences in side effects of the clinical trials among sex, race and age groups?
The tables below summarize two known vasopressor toxicities arrhythmias and vasoconstriction by demographic subgroup.
Table 3. Subgroup Analyses for Cardiac Arrhythmia-related AEs
| Demographic Parameter | GIAPREZA | Placebo |
|---|---|---|
| Overall | 51/163 (31%) | 60/158 (38%) |
| Sex | ||
| Women | 21/71 (30%) | 18/55 (33%) |
| Men | 30/92 (33%) | 42/103 (41%) |
| Age (years) | ||
| 31/90 (34%) | 26/77 (34%) | |
| ≥65 | 20/73 (27%) | 34/81 (42%) |
| Race | ||
| White | 37/135 (27%) | 45/122 (37%) |
| Non-White | 14/28 (50%) | 15/36 (42%) |
Table 4. Subgroup Analyses for Ischemia/Vasoconstriction AEs
| Demographic Parameter | GIAPREZA | Placebo |
|---|---|---|
| Overall | 17/163 (10%) | 16/158 (10%) |
| Sex | ||
| Women | 8/71 (11%) | 6/55 (11%) |
| Men | 9/92 (10%) | 10/103 (10%) |
| Age (years) | ||
| 9/90 (10%) | 7/77 (9%) | |
| ≥65 | 8/73 (11%) | 9/81 (11%) |
| Race | ||
| White | 16/135 (11.9%) | 14/122 (12%) |
| Non-White | 1/28 (3.6%) | 2/36 (6%) |
FDA Clinical Review
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved GIAPREZA based on evidence from one clinical trial of 321 critically ill patients who continue to have a dangerously low blood pressure (hypotension) despite receiving standard therapies.
The trial was conducted in 128 centers in the United States, Europe, Australia and New Zealand.
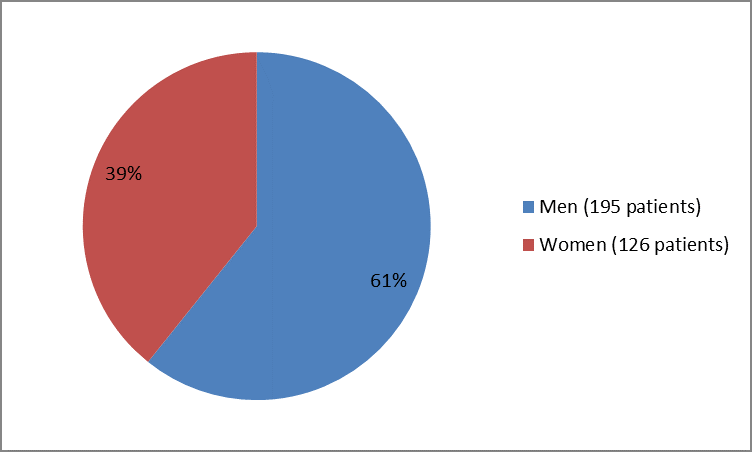
Figure 1 summarizes how many men and women were in the clinical trial.
Figure 1. Baseline Demographics by Sex
FDA Clinical review
Figure 2 and Table 1 summarize the percentage of patients by race in the clinical trial.
Figure 2. Baseline Demographics by Race
FDA Clinical review
Table 1. Baseline Demographics by Race
Race | Number of Patients | Percentage |
|---|---|---|
White | 257 | 80 |
Black or African American | 33 | 10 |
Asian | 13 | 4 |
American Indian or Alaska Native | 1 | less than 1 |
Native Hawaiian or other Pacific Islander | 1 | less than 1 |
Other | 16 | 5 |
FDA Clinical review
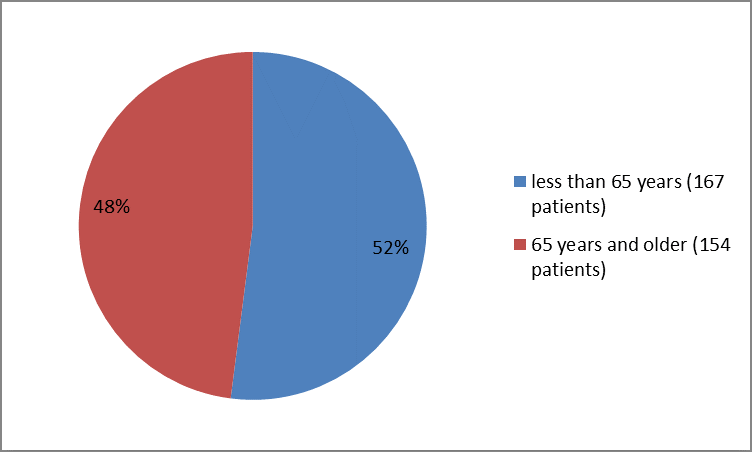
Figure 3 summarizes the percentage of patients by age in the clinical trial
Figure 3. Baseline Demographics by Age
FDA Clinical review
Who participated in the trials?
The table below summarizes demographics of patients (safety population) in clinical trial.
Table 5. Demographics of Safety Population
| Demographic Parameters | GIAPREZA | Placebo | Total |
|---|---|---|---|
| Sex | |||
| Men | 92 (56%) | 103(65%) | 195 (61%) |
| Women | 71 (44%) | 55 (35%) | 126 (39%) |
| Age | |||
| Mean years (SD) | 62 (16) | 63 (15) | 62 (15) |
| Median (years) | 63 | 65 | 64 |
| Min, max (years) | 22-89 | 22-89 | 22-89 |
| Age Group | |||
| ≥ 17 - | 90 (55%) | 77 (49%) | 167 (52%) |
| ≥ 65 years | 73 (45%) | 81 (51%) | 154 (48%) |
| >65 - | 32 (20%) | 39 (25%) | 71 (22%) |
| ≥ 75 years | 41 (25%) | 42 (27%) | 83 (26%) |
| Race | |||
| White | 135 (83%) | 122 (77%) | 257 (81%) |
| Black or African American | 14 (9%) | 19 (12%) | 33 (10%) |
| Asian | 5 (3%) | 8 (5%) | 13 (4%) |
| American Indian or Alaska Native | 1 (0.6%) | 0 | 1 (0.3%) |
| Native Hawaiian or Other Pacific Islander | 0 | 1 (0.6%) | 1 (0.3%) |
| Other | 8 (5%) | 8 (5%) | 16 (5%) |
| Ethnicity | |||
| Hispanic or Latino | 10 (6%) | 7 (4%) | 17 (5%) |
| Not Hispanic or Latino | 153 (94%) | 151 (96%) | 304 (95%) |
| Region | |||
| United States/Canada | 116 (71%) | 120 (76%) | 236 (74%) |
| Rest of the World | |||
| Europe | 19 (12%) | 14 (9%) | 33 (10%) |
| Australia/New Zealand | 28 (17%) | 24 (15%) | 52 (16%) |
FDA Clinical review
How were the trials designed?
There was one trial that evaluated the benefits and side effects of GIAPREZA.
Critically ill patients who did not adequately respond to standard therapies were randomly assigned to receive infusion of either GIAPREZA or placebo during treatment for shock in an intensive care unit. Neither the patients nor the health care providers knew which treatment was being given until after the trial was completed.
The benefit of GIAPREZA was measured by the proportion of patients who achieved the target blood pressure after 3 hours of treatment with GIAPREZA or placebo. During this period, the dose of other drugs used to raise blood pressure was kept constant. Target blood pressure was either a prespecified blood pressure or a prespecified increase in blood pressure to assure adequate circulation to vital organs.
How were the trials designed?
There was one randomized, multicenter, double-blind, placebo controlled trial that evaluated the safety and efficacy of GIAPREZA.
Enrolled patients were adults with septic or other distributive who remained hypotensive despite fluid and vasopressor therapy.
The primary endpoint was percentage of patients who achieved either a MAP ≥ 75 mmHg or a ≥ 10 mmHg increase in MAP without an increase in baseline vasopressor therapy at 3 hours.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.