Drug Trials Snapshots: BAXDELA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the BAXDELA Prescribing Information for complete information.
BAXDELA (delafloxacin)
(Bax-de’-lah)
Melinta Therapeutics, Inc.
Approval date: June 19, 2017
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
BAXDELA is an antibacterial medicine used to treat adult patients with bacterial skin infections known as acute bacterial skin and skin structure infections (ABSSSI) caused by certain bacteria.
How is this drug used?
BAXDELA is given by a healthcare professional using a needle placed in a vein (known as intravenous or IV infusion) over 60 minutes two times a day. BAXDELA can also be taken as a tablet two times a day for 5 to 14 days.
What are the benefits of this drug?
BAXDELA worked similarly to the standard antibacterial combination therapy of vancomycin and aztreonam in stopping the spread of ABSSSI.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes the efficacy results of the individual trials. In both trials, the primary endpoint was the proportion of patients who achieved an objective clinical response, i.e., reduction in lesion size of at least 20%, at 48 to 72 hours after initiating treatment. For the primary analysis, a conclusion of noninferiority is made if the lower bound of the 2-sided 95% confidence interval (CI) for the difference in the objective clinical response rates (delafloxacin – control) exceeds -10%. Efficacy results are presented in Intent-to –Treat (ITT) population.
Table 2. Clinical Response at 48–72 hours* in the ITT Population with ABSSSI in Trial 1 and Trial 2
| Trial | BAXDELA IV | Vancomycin+Aztreonam IV | Treatment Difference** (2-sided 95% CI) |
|---|---|---|---|
| Trial 1 | |||
| Total ,n | 331 | 329 | |
| Responder, n (%) | 259 (78.2%) | 266 (80.9%) | -2.6 (-8.8, 3.6) |
| BAXDELA IV and oral | Vancomycin+Aztreonam IV | Treatment Difference** (2-sided 95% CI) |
|
| Trial 2 | |||
| Total ,n | 423 | 427 | |
| Responder, n (%) | 354 (83.7%) | 344 (80.6%) | 3.1 (-2.0, 8.3) |
CI = Confidence Interval; ITT = Intent to Treat and includes all randomized patients
* Objective clinical response was defined as a 20% or greater decrease in lesion size as determined by digital planimetry of the leading edge of erythema at 48 to 72 hours after initiation of treatment without any reasons for failure (less than 20% reduction in lesion size, administration of rescue antibacterial therapy, use of another antibacterial or surgical procedure to treat for lack of efficacy, or death).
Missing patients were treated as failures.
**Treatment difference, expressed as percentage, and CI based on Miettinen and Nuriminen method without stratification.
BAXDELA Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: BAXDELA worked similarly in men and women.
- Race: Most of the patients were White. Differences in how well the drug worked among races could not be determined because of the small number of patients in other races.
- Age: BAXDELA worked similarly in patients above and below age 65.
Were there any differences in how well the drug worked in clinical trials among sex, race and age groups?
The tables below summarize subgroup differences based on the primary endpoint analyses for each trial separately.
Table 3. Objective Clinical Response at 48 to 72 hours by Age, Sex, Race and Geographic Region-Trial 1 (ITT population)
| Demographic Characteristic | BAXDELA IV N=331 |
Control N=329 |
Difference (95% CI) |
|---|---|---|---|
| Overall | 259/331 (78.2) | 266/329 (80.9) | -2.6 (-8.8, 3.6) |
| Age in years | |||
| <> | 242/305 (79.3) | 253/309 (81.9) | -2.5 (-8.8, 3.7) |
| ≥65 | 17/26 (65.4) | 13/20 (65.0) | 0.4 (-27.4, 28.2) |
| Sex | |||
| Women | 100/125 (80.0) | 95/120 (79.2) | 0.8 (-9.3, 11.1) |
| Men | 159/206 (77.2) | 171/209 (81.8) | -4.6 (-12.5, 3.2) |
| Race | |||
| Black or African American | 23/27 (85.2) | 15/19 (79.0) | 6.2 (-22.6,34.5)* |
| White | 231/297 (77.8) | 247/304 (81.3) | -3.5 (-10.0,3.0) |
| Other | 5/7 (71.4) | 4/6 (66.7) | 4.8 (-49.7, 56.0) |
| Geographic Region | |||
| United States | 213/268 (79.5) | 226/274 (82.5) | -3.0 (-9.7, 3.6) |
| Europe | 46/63 (73.0) | 40/55 (72.7) | 0.3 (-15.7, 16.6) |
| Other | -- | -- | -- |
Table 4. Objective Clinical Response at 48 to 72 hours by Age, Sex, Race and Geographic Region-Trial 2 (ITT population)
| Demographic Characteristic |
BAXDELA |
Control N=427 |
Difference (95% CI) |
|---|---|---|---|
| Overall | 354/423 (83.7) | 344/427 (80.6) | 3.1 (2.0,8.3) |
| Age in years | |||
| <> | 287/338 (84.9) | 285/346 (82.4) | 2.5 (-3.0, 8.1) |
| ≥65 | 67/85 (78.8) | 59/81 (72.8) | 6.0 (-7.1, 19.0) |
| Sex | |||
| Women | 132/161 (82.0) | 118/151 (78.2) | 3.8 (-5.1, 12.8) |
| Men | 222/262 (84.7) | 226/276 (81.9) | 2.9 (-3.5, 9.2) |
| Race | |||
| Black or African American | 9/13 (69.2) | 18/18 (100) | -30.8(-61.4, 4.5)* |
| White | 299/348 (85.9) | 290/355 (81.7) | 4.2 (-1.2, 9.7) |
| Other | 46/62 (74.2) | 36/54 (66.7) | 7.5 (-9.1, 24.1) |
| Geographic Region | |||
| United States | 180/202 (89.1) | 170/196 (86.7) | 2.4 (-4.1, 9.0) |
| Europe | 132/165 (80.0) | 134/173 (77.5) | 2.5 (-6.3, 11.3) |
| Other | 42/56 (75.0) | 40/58 (69.0) | 6.0 (-10.6, 22.3) |
*CI based on exact methods; otherwise using Miettinen and Nuriminen method.
Adapted from FDA Statistical review
What are the possible side effects?
BAXDELA like other antibacterial medicines called fluoroquinolones may cause the following serious side effects: tendon rupture, nerve damage (peripheral neuropathy) and certain central nervous system effects.
Serious side effects in patients treated with BAXDELA in clinical trials include allergic reactions and colon infection caused by Clostridium difficile
The most common side effects in patients treated with BAXDELA in clinical trials were nausea, diarrhea, headache, increase in certain liver tests, and vomiting.
What are the possible side effects (results of trials used to assess safety)?
Below is the summary of the most common adverse reactions observed in ≥ 2% patients treated with BAXDELA in pooled safety population from the trials.
Table 5. Selected Adverse Reactions Occurring in ≥ 2% of Patients Receiving BAXDELA in the Pooled Clinical Trials
|
Adverse Reactions |
BAXDELA |
Vancomycin/aztreonam |
|---|---|---|
|
Nausea |
8% |
6% |
|
Diarrhea |
8% |
3% |
|
Headache# |
3% |
6% |
|
Transaminase Elevations* |
3% |
4% |
|
Vomiting |
2% |
2% |
# The data are not an adequate basis for comparison of rates between the study drug and the active control.
*Pooled reports include hypertransaminasaemia, increased transaminases, and increased ALT and AST.
BAXDELA Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of nausea and diarrhea side effects was similar in men and women.
- Race: Most of the patients were White. Differences in the occurrence of nausea and diarrhea among races could not be determined because of the small number of patients in other races.
- Age: The occurrence of nausea and diarrhea side effects was similar in patients above and below age 65.
Were there any differences in side effects of the clinical trials among sex, race and age groups?
Tables below summarize the occurrence of the two most common adverse reactions by subgroups based on pooled safety population.
Table 6. Subgroup Analysis by Sex
| BAXDELA N=741 |
Vancomycin/aztreonam N = 751 |
|
|---|---|---|
| Men , x/n (% ) | ||
| Diarrhea | 36/459 (7.8) | 15/483 (3.1) |
| Nausea | 30/459 (6.5) | 25/483 (5.2) |
| Women , x/n (% ) | ||
| Diarrhea | 22/282 (7.8) | 9/268 (3.4) |
| Nausea | 26/282 (9.2) | 22/268 (8.2) |
Table 7. Subgroup Analysis by Race
| BAXDELA N=741 | Vancomycin/aztreonam N = 751 | |
|---|---|---|
| White , x/n (% ) | ||
| Diarrhea | 52/636 (8.2) | 19/656 (2.9) |
| Nausea | 51/636 (8.0) | 41/656 (6.3) |
| Black or African American , x/n (% ) | ||
| Diarrhea | 3/38 (7.9) | 2/36 (5.6) |
| Nausea | 2/38 (5.3) | 4/36 (11.1) |
Table 8. Subgroup Analysis by Age
| BAXDELA N=741 | Vancomycin/aztreonam N = 751 | |
|---|---|---|
| ≤65 years , x/n (% ) | ||
| Diarrhea | 52/640 (8.1) | 20/656 (3.0) |
| Nausea | 54/640 (8.4) | 47/656 (7.2) |
| >65 years , x/n (% ) | ||
| Diarrhea | 6/101 (5.9) | 4/95 (4.2) |
| Nausea | 2/101 (2.0) | 0 |
x/n=number of patients with adverse reaction in subpopulation sample
Clinical trial data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved BAXDELA based on evidence from two clinical trials of 1510 patients with skin infections. The trials were conducted in the United States, Europe, Latin America and Asia.
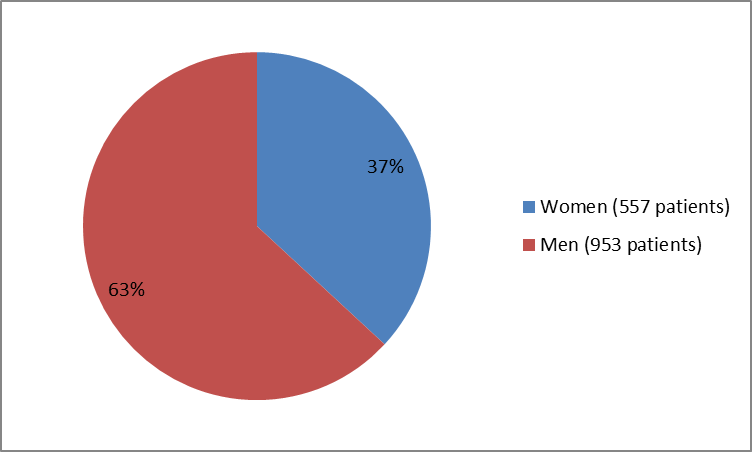
Figure 1 summarizes how many men and women were in the clinical trials.
Figure 1. Baseline Demographics by Sex
Clinical trial data
Figure 2 and Table 1 summarize the percentage of patients by race in the clinical trials.
Figure 2. Baseline Demographics by Race
Clinical trial data
Table 1. Baseline Demographics by Race
|
Race |
Number of Participants |
Percentage |
|---|---|---|
|
White |
1304 |
86 |
|
Black or African American |
77 |
5 |
|
All Other |
129 |
9 |
Clinical trial data
Figure 3 summarizes the percentage of patients by age in the clinical trials.
Figure 3. Baseline Demographics by Age
Clinical trial data
Who participated in the trials?
The table below summarizes demographics of patients in clinical Trials 1 and 2.
Table 9. Baseline and Demographics (ITT Population)
|
Trial 1 Demographic BAXDELA |
Trial 2 BAXDELA |
|---|---|
|
Age Group, n (%) |
|
|
Age |
|
|
Sex, n (%) |
|
|
Race, n (%) |
|
|
Geographic Region, n (%) |
|
Adapted from FDA Statistical review
How were the trials designed?
There were two trials that established the benefits and side effects of BAXDELA. Trials enrolled adult patients with acute bacterial skin and skin structure infections.
In Trial 1, patients were randomly assigned to receive either BAXDELA IV every 12 hours or a comparator ( vancomycin and aztreonam) IV every 12 hours, a treatment regimen that is acceptable therapy for ABSSSI. Neither the patients nor the health care providers knew which injection was being given until after the trial was completed.
In Trial 2, patients started treatment with BAXDELA IV or comparator (vancomycin and aztreonam) IV every 12 hours. After 3 days (total of 6 doses) patients who received BAXDELA IV, continued treatment with BAXDELA tablets (one pill every 12 hours) while patients in the comparator group continued with their IV treatment. Neither the patients nor the health care providers knew which treatment was being given until after the trial was completed.
The planned treatment duration was 5 to 14 days for both trials.
The benefit of BAXDELA was measured by the proportion of patients who achieved an improvement (reduction in infection lesion size of at least 20%), at 48 to 72 hours after the initiation of the treatments.
How were the trials designed?
There were two randomized, multicenter, double-blind, double-dummy, non-inferiority trials that evaluated safety and efficacy of BAXDELA in patients with acute bacterial skin and skin structure infections (ABSSSI). Enrolled patients had the following infections: cellulitis, wound infection, major cutaneous abscess, or burn infection.
In Trial 1, patients were randomized to receive BAXDELA or vancomycin plus aztreonam via intravenous infusion every 12 hours.
In Trial 2, patients received BAXDELA via intravenous infusion every 12 hours for 6 doses and then made a mandatory switch to oral BAXDELA every 12 hours. Control arm received vancomycin plus aztreonam via intravenous infusion every 12 hours.
In both trials, the primary efficacy endpoint was objective clinical response at 48 to 72 hours post initiation of treatment, defined as a 20% or greater decrease in lesion size as determined by digital planimetry of the leading edge of erythema.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.