2023 FDA Science Forum
Assessment of factors that contribute to inflammatory toxicities during CAR-T cell therapy
- Authors:
- Center:
-
Contributing OfficeCenter for Biologics Evaluation and Research
Abstract
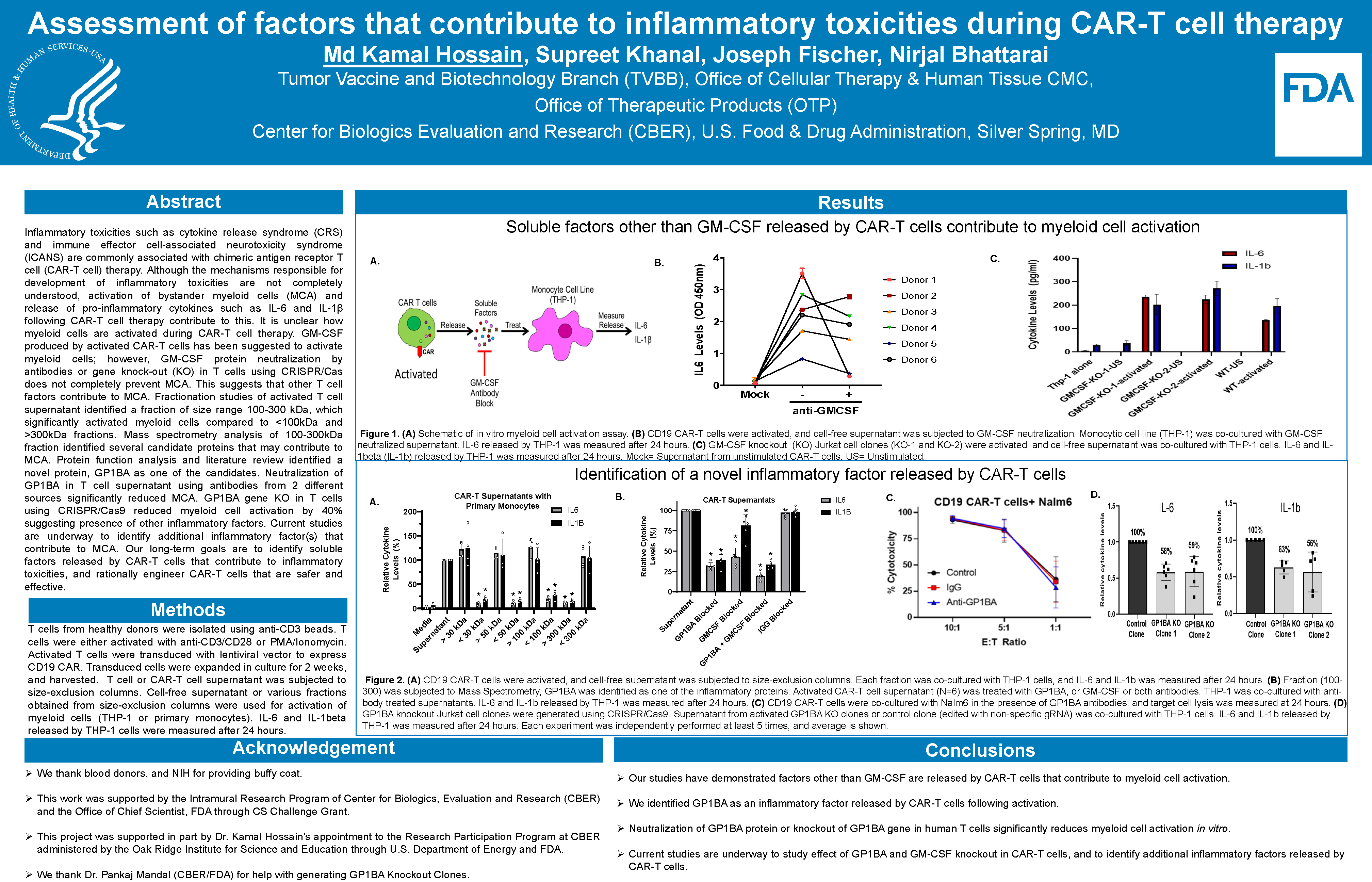
Inflammatory toxicities such as cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) are commonly associated with chimeric antigen receptor T cell (CAR-T cell) therapy. Although the mechanisms responsible for development of inflammatory toxicities are not completely understood, activation of bystander myeloid cells (MCA) and release of pro-inflammatory cytokines such as IL-6 and IL-1β following CAR-T cell therapy contribute to this. It is unclear how myeloid cells are activated during CAR-T cell therapy. GM-CSF produced by activated CAR-T cells has been suggested to activate myeloid cells; however, GM-CSF protein neutralization by antibodies or gene knock-out (KO) in T cells using CRISPR/Cas does not completely prevent MCA. This suggests that other T cell factors contribute to MCA. Fractionation studies of activated T cell supernatant identified a fraction of size range 100-300 kDa, which significantly activated myeloid cells compared to <100kDa and >300kDa fractions. Mass spectrometry analysis of 100-300kDa fraction identified several candidate proteins that may contribute to MCA. Protein function analysis and literature review identified a novel protein, GP1BA as one of the candidates. Neutralization of GP1BA in T cell supernatant using antibodies from 2 different sources significantly reduced MCA. However, GP1BA gene KO in T cells did not prevent MCA. Furthermore, recombinant GP1BA protein did not cause MCA. Interestingly, addition of anti-GP1BA antibodies in the supernatant from GP1BA KO T cells also prevented MCA. So far, these data suggest that T cell derived GP1BA does not contribute to MCA but GP1BA antibodies cross-react with an unidentified T cell-derived inflammatory factor, which contribute to MCA. Current studies are underway to identify inflammatory factor(s) that cross-react with GP1BA antibodies. Our long-term goals are to identify soluble factors released by CAR-T cells that contribute to inflammatory toxicities, and rationally engineer CAR-T cells that are safer and effective.
Download the Poster (PDF; 0.40 MB)
