COMPANY ANNOUNCEMENT
Tris Pharma Issues Voluntary Nationwide Recall of Infants’ Ibuprofen Concentrated Oral Suspension, USP (NSAID) 50 mg per 1.25 mL, Due to Potential Higher Concentrations of Ibuprofen
This recall has been completed and FDA has terminated this recall.
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
Over-the-Counter Drugs - Reason for Announcement:
-
Recall Reason DescriptionDue to Potential Higher Concentrations of Ibuprofen
- Company Name:
- Tris Pharma
- Brand Name:
-
Brand Name(s)Family Wellness, CVS, Equate
- Product Description:
-
Product DescriptionInfants’ Ibuprofen
Company Announcement
Tris Pharma, Inc. has voluntarily recalled three (3) lots of Infants’ Ibuprofen Concentrated Oral Suspension, USP (NSAID) 50 mg per 1.25 mL, to the retail level. The recalled lots of the product have been found to potentially have higher concentrations of ibuprofen.
There is a remote possibility that infants, who may be more susceptible to a higher potency level of drug, and therefore may be more vulnerable to permanent NSAID-associated renal injury. Adverse effects that may be experienced are nausea, vomiting, epigastric pain, or more rarely, diarrhea. Tinnitus, headache and gastrointestinal bleeding are also possible adverse effects. To date, Tris Pharma, Inc. has not received any reports of adverse events related to the lots of product that are the subject of this recall.
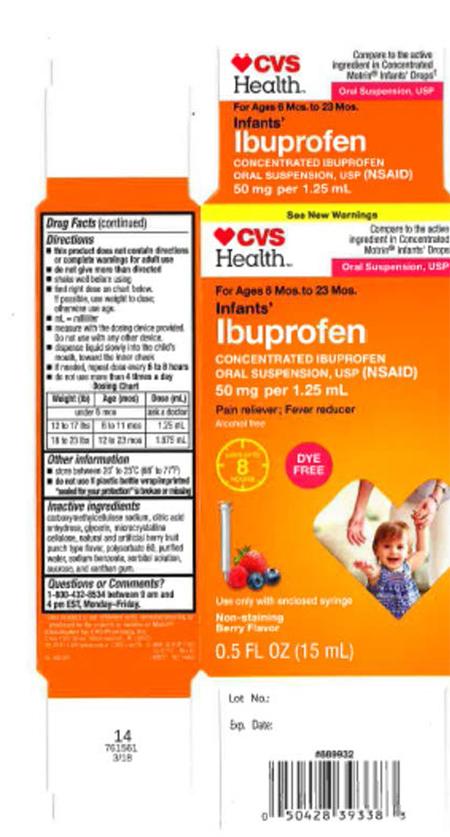
The product is used as a pain reliever/fever reducer and was packaged in 0.5 oz. bottles for the recalled lots listed below:
|
NDC; |
LOT; |
EXPIRATION; |
DESCRIPTION; |
COMPANY; |
|---|---|---|---|---|
| 49035-125-23 | 00717009A 00717015A 00717024A |
02/19 04/19 08/19 |
Equate: Infants’ Ibuprofen Concentrated Oral Suspension, USP (NSAID), 50 mg per 1.25 mL, 0.5 oz. bottle |
Wal-Mart Stores Inc |
| 59779-925-23 | 00717024A | 08/19 | CVS Health: Infants’ Ibuprofen Concentrated Oral Suspension, USP (NSAID), 50 mg per 1.25 mL, 0.5 oz. bottle |
CVS Pharmacy |
| 55319-250-23 | 00717024A | 08/19 | Family Wellness: Infants’ Ibuprofen Concentrated Oral Suspension, USP (NSAID), 50 mg per 1.25 mL, 0.5 oz. bottle |
Family Dollar Services Inc. |
Tris Pharma, Inc. sold the affected lots of Ibuprofen Concentrated Oral Suspension, USP (NSAID) 50 mg per 1.25 mL to one customer, which distributed the lots into the US market. Tris Pharma, Inc. has notified its customer by urgent recall notice and is arranging for the return of the recalled product.
Wholesalers and retailers of the product should stop further distribution of the affected lots of Ibuprofen Concentrated Oral Suspension, USP (NSAID) 50 mg per 1.25 mL, which are being recalled.
Consumers with questions regarding this recall can contact Tris Customer Service at 732-940-0358 (Monday through Friday, 8:00am ET- 5:00pm PT) or via email at Customer Service Email . Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
About Tris Pharma
Tris Pharma is a fully integrated pharmaceutical company focused on the development of innovative medicines that address unmet patient needs. Using its proprietary technology platform, LiquiXR®, Tris has pioneered the delivery of sustained release in the liquid, chewable, orally disintegrating tablet, and other dosage forms that benefits a wide variety of patients and their unique needs. Tris' Nobuse™ technology provides abuse deterrence for opioids and other abuse-prone drugs. Tris’ research, manufacturing and commercial facilities are located in Central New Jersey. For more information, please visit www.trispharma.com.