COMPANY ANNOUNCEMENT
Olympia Pharmacy Issues Voluntary Nationwide Recall of Seven Compounded Products Due to Being Out-of-Specification
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason DescriptionProducts are out of specification
- Company Name:
- Olympia Pharmacy
- Brand Name:
-
Brand Name(s)Olympia Pharmaceuticals
- Product Description:
-
Product DescriptionCompounded Injectables
Company Announcement
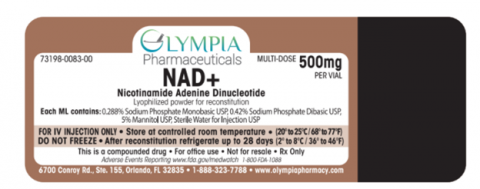
Olympia Pharmacy is voluntarily recalling 11 specific lots of Trimix Formulas F-9, T-105, SB-4, Sermorelin, Sincalide, Hydroxocobalamin, and NAD, compounded injectables to the consumer level. These compounded products were found to be out-of-specification.
Risk Statement: Administration of subpotent Hydroxocobalamin in infants, pregnant/breastfeeding women, and elderly populations are at risk for vitamin B12 deficiency and there is a reasonable probability they could experience adverse events including muscle weakness, neurological peripheral neuropathic numbness or pain, vision loss, and psychiatric disorders (depression, memory loss). Additionally, injectable compounded products, found to contain more or less drug product than the labeled strength or which reconstitute at a different rate than intended, may result in either too much or too little medication being administered. This could result in lower-than-expected effectiveness of the drug or unintended adverse side effects.
Olympia Pharmacy has not received any reports or concerns from patients relating to the safety of the recalled sterile compounded products, and no patients have reported any adverse events attributed to any of the recalled sterile compounded products.
The compounded products being recalled are typically prescribed by medical professionals for age management, erectile dysfunction, vitamin deficiencies, and for diagnostic imaging of the gallbladder. The affected lots include the following lot numbers and expiration dates listed below. The product can be identified by reading the lot number in the black strike zone of the label and was distributed to patients and health clinics.
| Drug | Vial Size | Lot | Best Use Date |
|---|---|---|---|
| NAD | 500mg vial | C41008 | 3/8/22 |
| NAD | 500mg vial | D24005 | 4/5/22 |
| Sincalide | 5 mcg vial | D24001 | 4/1/22 |
| Trimix Formula F9 | 10 ml vial | D41C19 | 4/19/22 |
| Sermorelin Acetate 9 mg | 9 mg vial | D44026 | 4/26/22 |
| Sermorelin Acetate 9 mg | 9 mg vial | F42104 | 6/4/22 |
| Trimix T-105, | 5 ml vial | E41F10 | 5/10/22 |
| Trimix T-105, | 10 ml vial | E41G10 | 5/10/22 |
| Trimix SB-4 | 5 ml vial | E41C18 | 5/18/22 |
| Trimix SB-4 | 10 ml vial | E41D18 | 5/18/22 |
| Hydroxocobalamin 1mg/ml | 30 ml vial | E47025 | 5/21/22 |
Olympia Pharmacy is notifying its customers by mail and is arranging for return and replacement of all recalled compounded products. Patients and health clinics that have any of the listed compounded products which are being recalled should stop using and return to Olympia Pharmacy.
Consumers with questions regarding this recall can contact Olympia Pharmacy by phone at 407-250-4000 or e-mail clientservices@olympiapharmacy.com Monday through Friday from 9 am to 6 pm Eastern Standard Time.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Company Contact Information
- Consumers:
- Recall Coordinator
- 407-250-4000
- clientservices@olympiapharmacy.com