Global Approach to Software as a Medical Device
The International Medical Device Regulators Forum (IMDRF) Software as a Medical Device Working Group (WG) published a possible risk categorization framework for Software as a Medical Device. The recommendations provided in this document allow manufactures and regulators to more clearly identify risk categories of Software as a Medical Device based on how the output of a Software as a Medical Device is used for healthcare decisions in different healthcare situations or conditions.
Possible IMDRF Framework for Risk Categorization of Software as a Medical Device
The Software as a Medical Device risk categorization framework has four categories (I, II, III, and IV). These categories are based on the levels of impact on the patient or public health where accurate information provided by the Software as a Medical Device to treat or diagnose, drive or inform clinical management is vital to avoid death, long-term disability or other serious deterioration of health, mitigating public health. The Level IV category is Software as a Medical Device with the highest impact on the patient or public health and Level I is the lowest.
| State of Health care situation or condition | Significance of information provided by SaMD to health care decision | ||
|---|---|---|---|
| Treat or diagnose | Drive clinical management | Inform clinical management | |
| Critical | IV | III | II |
| Serious | III | II | I |
| Non-serious | II | I | I |
International Medical Device Regulators Forum Quality Management System for Software as a Medical Device
The IMDRF Quality Management System for Software as a Medical Device (SaMD) framework helps manufacturers and international regulators attain a common understanding and vocabulary for the application of medical device quality management system requirements to Software as a Medical Device. It also complements the IMDRF Software as a Medical Device framework for risk categorization . It highlights clinical and technical considerations that are especially relevant to the unique features of Software as a Medical Device.
Good software quality and engineering practices need to be incorporated into the device's quality management system to assure medical device quality management principles are met. The IMDRF Software as a Medical Device framework provides harmonized quality management principles for the FDA, along with other regulators, to adopt based on their own regulatory framework, and are not regulations.
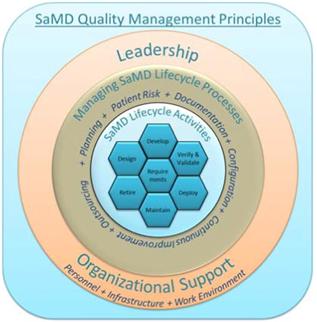
- An organizational support structure providing leadership, accountability, and governance with adequate resources to assure the safety, effectiveness, and performance of SaMD;
- A set of Software as a Medical Device lifecycle support processes that are scalable for the size of the organization and applied consistently across all realization and use processes (requirements management, design, development, verification and validation, deployment, maintenance, and decommissioning of the product); and,
- A set of realization and use processes that are scalable for the type of Software as a Medical Device and the size of the organization that take into account important elements required for assuring the safety, effectiveness, and performance of Software as a Medical Device.
International Medical Device Regulators Forum Software as a Medical Device: Clinical Evaluation
Health care decisions increasingly rely on information provided by Software as a Medical Device output, where these decisions can impact clinical outcomes and patient care. The FDA issued the Software as a Medical Device: Clinical Evaluation final guidance to describe an internally agreed upon understanding of clinical evaluation and principles for demonstrating the safety, effectiveness, and performance of Software as a Medical Device among regulators in the International Medical Device Regulators Forum. The guidance focuses on the activities Software as a Medical Device manufacturers can take to clinically evaluate their Software as a Medical Device.
This guidance provides the FDA with an initial framework to help further development of FDA's specific regulatory approaches and expectations for regulatory oversight. This guidance does not provide recommendations for FDA staff and industry to apply to specific regulatory situations, nor does it modify current regulatory expectations, including those for regulatory submissions, at this time.
For more information on FDA adoption of IMDRF documents as an FDA guidance document, please see the webpage about FDA's work in the International Medical Device Regulators Forum.
