SMALL ENTITY COMPLIANCE GUIDE
Small Entity Compliance Guide: Statement of Identity, Nutrition Labeling and Ingredient Labeling of Dietary Supplements January 1999
- Docket Number:
- FDA-2020-D-1959
- Issued by:
-
Guidance Issuing OfficeCenter for Food Safety and Applied Nutrition
The Food and Drug Administration (FDA) has prepared this guide in accordance with section 212 of the Small Business Regulatory Fairness Act. This guidance document represents the agency's current thinking on the statement of identity, nutrition labeling, and ingredient labeling of dietary supplements. It does not create or confer any rights for or on any person and does not operate to bind FDA or the public. An alternative approach may be used if such approach satisfies the requirements of the applicable statute, regulations, or both.
On October 25, 1994, the President signed the Dietary Supplement Health and Education Act (DSHEA). This law defines dietary supplements and requires that dietary supplements be clearly identified. Also, the law requires that nutrition information followed by ingredient information be stated on the labels of dietary supplements. The nutrition information is to list the dietary ingredients present in a supplement and to state the amounts of these ingredients. Additionally, when a supplement contains any material from a plant, the label must identify any part of a plant used.
Previously, only nutrients that have daily recommendations, such as 1000 mg for calcium, could be listed in the nutrition information. The new law allows dietary ingredients for which recommendations have not been established to be listed as long as the label indicates this fact.
Additionally, the new law allows information on sources to be included in the nutrition information. Previously, this information was given in the ingredient list. For example, if calcium is from calcium carbonate, the nutrition information can now state "calcium (as calcium carbonate)." When a source is listed in this manner, it does not have to be listed again in the ingredient information.
In response to DSHEA, FDA has amended its regulations with respect to dietary supplements in the FEDERAL REGISTER of September 23, 1997 (62 FR 49826). Manufacturers may comply immediately, but all labels applied to dietary supplements after March 23, 1999 are to be in compliance with these regulations.
To help consumers identify dietary supplements, the new regulations require that dietary supplement products have the term "supplement" in their name, e.g. vitamin C supplement. Additionally, to highlight the nature of these products, the nutrition information is titled "Supplement Facts."
As provided by DSHEA, the labels of dietary supplements are to list the names and amounts per serving of dietary ingredients. A serving of a dietary supplement is the amount recommended in one eating occasion. This information is stated at the top of the nutrition information following the words "Serving Size." Similar to the labels of conventional foods, FDA is requiring that the nutrients most important to the public health be listed. Other vitamins and minerals may be declared, but they must be declared when they are added for purposes of supplementation or when a claim is made about them. Dietary ingredients are to be listed in the order specified in the regulation and their amounts may immediately follow the names or be in a separate column. For uniformity, amounts are to be declared using the units of measurement specified in the regulation.
In addition to the names and amounts, FDA is requiring that the "% Daily Values" be listed. For example, the recommended daily amount for vitamin C is 60 mg. If a food contains this amount, the "% Daily Value" for vitamin C would be 100%. The "% Daily Values" on labels are intended for adults and children 4 or more years of age, unless stated otherwise.
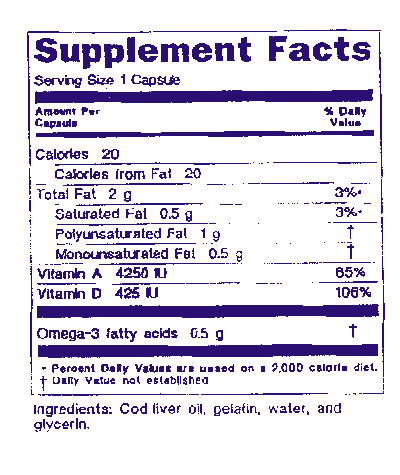
Following the listing of dietary ingredients that have recommendations, other dietary ingredients not having recommendations may be listed as long as this fact is indicated. Accordingly, the names and amounts of these dietary ingredients may be listed in the next section of the nutrition label with an asterisk in the "% Daily Value" column that refers to the footnote "Daily Value not established." The following sample label is presented for the purpose of illustration:
Other dietary ingredients are to be listed by their common names. Botanicals are to be listed in accordance with the standard terminology in the book Herbs of Commerce. In most cases, this reference also gives the corresponding Latin binomial for each common name. When the Latin name cannot be obtained from this reference, the label must state the Latin name of the botanical.
DSHEA provides that when the dietary ingredients in a supplement are considered to be a proprietary blend, just the total amount of the blend need be stated. In the absence of individual amounts, FDA requires that the dietary ingredients in a proprietary blend are to be listed in order of predominance by weight. These blends also are to be identified by the term "Proprietary Blend" or other appropriately descriptive term or fanciful name.
With respect to dietary supplements that are extracts, labeling requirements were amended in the FEDERAL REGISTER of June 5, 1998. For liquid extracts, the quantity may be listed by weight or by volume, the listing of starting material to the final volume of solvent is optional, and solvents present may be listed either in the nutrition label or in the ingredient statement. For dry extracts, FDA does not require identification of the solvent used to produce the extract.
FDA is requiring that the nutrition information be presented in a certain format that is similar to the presentation of nutrition information on all foods. Some of the major features of the format include use of the largest type size for the title, a hairline box around the nutrition information, bars that separate parts of the nutrition label, lines that separate the listing of dietary ingredients, a single easy-to-read type style, all black or one color type on a white or other neutral contrasting background (whenever practical), upper and lower case letters except on very small packages, at least one point leading, and letters that do not touch.
With respect to the type size of the information presented in the nutrition label, the minimum size required depends on the total surface area available for labeling. Large packages (those having over 40 square inches) are to have a minimum type size of 8 point, except that no smaller than 6 point may be used for column headings and footnotes. Small packages (those having less than 12 square inches) are to have a minimum type size no smaller than 4.5 point. Intermediate-sized packages (from 12 to 40 square inches) are to have a minimum of 6 point, except that a minimum of 4.5 point may be used on packages that have less than 20 square inches and more than 8 dietary ingredients to be listed and on packages that have 20 to 40 square inches and more than 16 dietary ingredients.
FDA has determined that the compliance requirements in §101.9(g) for conventional foods are generally applicable to dietary supplements. It should be noted that supplements should contain at least the amounts of dietary ingredients that are declared, except that for a dietary ingredient that is naturally-occurring, a label is not considered misbranded when an FDA analysis finds 80% of the amount declared on the label. Reasonable excesses of most dietary ingredients are acceptable within current good manufacturing practice, except that no more than a 20% excess is allowed for calories, sugars, total fat, saturated fat, cholesterol, or sodium.
FDA has three types of exemptions from nutrition labeling that are available for food products whose labels do not make nutrient content or health claims. Dietary supplement products that qualify for these exemptions do not have to have a "Supplement Facts" panel. One type of exemption is for products that are sold by small businesses that meet the criteria in §101.9(j)(1) (under $50,000 worth of food sales or under $500,000 worth of total sales to consumers). Another exemption is for products that are "low-volume" as defined in §101.9(j)(18). The last exemption applies to products shipped in bulk that are not distributed to consumers in such form as described in §101.9(j)(9).
FDA also has special labeling provisions for food products that are applicable to dietary supplements. This final rule states in §101.36(i) that dietary supplements are subject to the special provisions for foods for infants and children, foods in small and intermediate-sized packages, foods in multiunit containers, and foods sold in bulk products in §101.9(j). Firms in need of other special allowances may write to the FDA for permission.
Related Information
Submit Comments
You can submit online or written comments on any guidance at any time (see 21 CFR 10.115(g)(5))
If unable to submit comments online, please mail written comments to:
Dockets Management
Food and Drug Administration
5630 Fishers Lane, Rm 1061
Rockville, MD 20852
All written comments should be identified with this document's docket number: FDA-2020-D-1959.