Impact Story: Supporting Drug Development Through Physiologically Based Pharmacokinetic Modeling
CDER clinical pharmacologists are helping the drug development community use computational models to achieve safe and effective dosing recommendations for patients who are taking more than one drug.
The 16th-century Swiss physician and philosopher Paracelsus once said, “All things are poison, and nothing is without poison. The dosage makes it either a poison or a remedy.” This quote underscores the fact that developing the right dosing recommendations for a drug is a critical step in preventing drug toxicities while ensuring effectiveness. These recommendations depend on knowledge of a drug’s pharmacokinetics—the time course of its absorption, distribution, metabolism, and excretion—and its pharmacodynamics—the relationship between drug concentration at the site of action and the resulting effect. The pharmacokinetics and pharmacodynamics of a new drug are studied in clinical trials, and it is the evidence from these trials that forms the basis for determining its correct dosing.
However, many factors may influence a drug’s pharmacokinetics, making it difficult or impossible to provide direct evidence for its safe and effective use in all patients. These factors include age, sex, the presence of various comorbidities and organ dysfunctions, and other drugs a patient may be using. Trying to address all of these factors in clinical studies would require huge, expensive trials and could severely impede drug development.
Mathematical models called physiologically based pharmacokinetic (PBPK) models make it possible to simulate clinical trials that support dosing recommendations, as well as improve the design of real trials. This effort, which is a major focus of CDER research, has recently resulted in significant benefits to the drug development community.
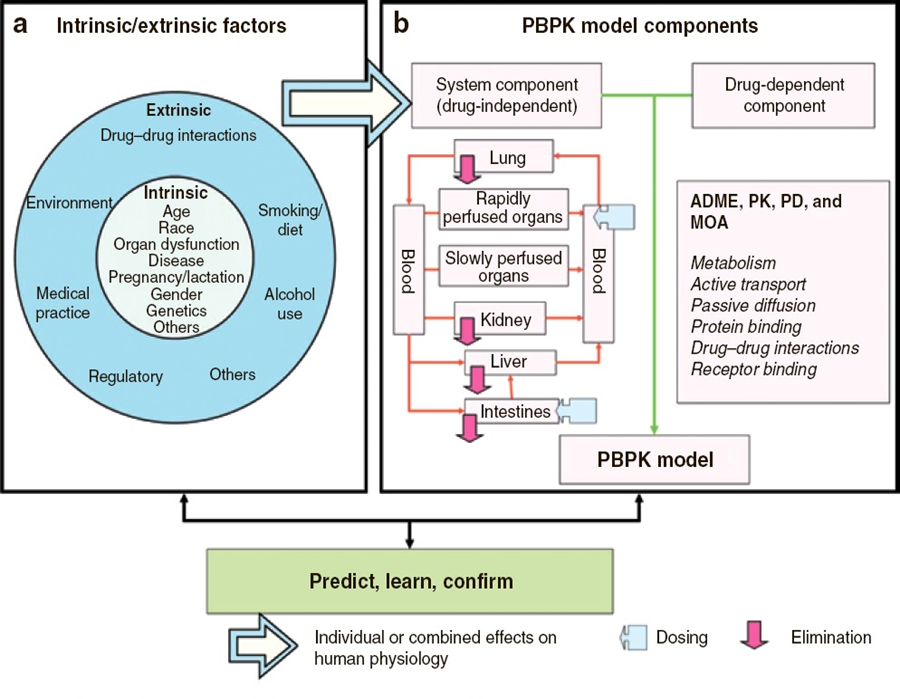
Figure 1. A PBPK model (panel b) is a set of equations that incorporate numerical values (parameters) describing physiology, anatomy, and drug properties to simulate the changes of drug concentrations in organs and tissues over time. The model includes both a drug‐dependent component and a drug‐independent system component. The latter is based on knowledge of body fluid dynamics (e.g., blood and urine flow), tissue size and composition, abundance and distribution of drug receptors, drug‐metabolizing enzymes, and membrane transporters in various organ and tissue compartments. The effects of both intrinsic factors (e.g., age and genetics) and extrinsic factors (e.g., the presence of additional medications that cause drug–drug interactions; panel a) can be assessed by incorporating values that represent specific populations and/or scenarios. PBPK = physiologically based pharmacokinetic; PK = pharmacokinetic; PD = pharmacodynamic; ADME = absorption, distribution, metabolism, and excretion; and MOA = mechanism of action. Figure from Zhao, P., et al., 2011, Clinical Pharmacology & Therapeutics, 89 (2): 259-267.
PBPK Models to Mechanistically Predict Drug Pharmacokinetics
PBPK modeling is grounded in our knowledge of human physiology. The equations that make up a PBPK model (Figure 1) describe the time course of absorption, distribution, metabolism, and elimination of one or more drugs in the body. Commonly accepted values for anatomical or physiological parameters such as blood flow, tissue composition, and enzyme abundance are entered into these equations. Also included are drug-specific values, such as tissue-to-plasma concentration ratios and how rapidly the drug is broken down by a given enzyme.
In theory, this approach allows us to make predictions based on our knowledge from previous trials and the data from in vitro and pre-clinical studies. For example, based on a PBPK model, we could predict the drug concentrations achieved—in plasma at particular times—that would result from a specific dose in a patient from a population for which clinical data was unavailable. We could then potentially extend the drug’s indications to that group of patients. In reality, gaps in our knowledge often prevent us from using this approach, because the prediction errors are high. To improve the accuracy of the model’s predictions, we need to address certain challenges, including the difficulty in measuring some of the physiologic parameters in both healthy adults and specific populations, the lack of relevance of in vitro and preclinical systems to humans, and incomplete understanding of the drugs themselves.
CDER scientists and researchers are seeking to close these gaps and allow PBPK models to be incorporated in drug development. For example, by analyzing the performance of models submitted to the FDA, CDER researchers have shown that the PBPK modeling approach can inform pediatric clinical trials and potentially be used to expedite drug development for children through a learn-and-confirm approach, which allows newly available data to be used to continuously develop and improve models.
In another example, clinical pharmacologists at CDER are looking for ways to support drug development and evaluation for patients with chronic kidney disease (CKD). They developed PBPK models for seven drugs cleared from the body by kidney proteins called organic anion transporters. By incorporating the effects of CKD on physiology and comparing the modeling results to clinical data, the researchers showed that these PBPK models reasonably predict the PK of those drugs in CKD patients. These and other investigations by CDER scientists improve the predictive performance of PBPK models so that they may be used to support regulatory decision-making about safety and effectiveness in patients for whom data are lacking. The ultimate goal of these efforts is to ensure that all patients who can benefit from new drug therapies receive them.
PBPK Modeling to Predict and Understand Drug–Drug Interactions, Minimize Clinical Trial Burden, and Develop Dosing Recommendations in Patients Taking Additional Drugs
One of the greatest challenges in evaluating a new drug is that the presence of an additional drug may alter its pharmacokinetics or action, a phenomenon known as drug–drug interaction (DDI). A common type of DDI occurs when the “perpetrator” drug affects the metabolism of the “victim” drug by changing the activity of the enzyme or enzymes that break down the victim drug. These metabolizing enzymes are often members of the extensive family of cytochrome P450 (CYP) enzymes, located in the liver and other organs, that are responsible for much of the drug’s detoxification and removal from the body. When an enzyme’s activity is reduced, the concentration of the victim drug can be increased. Alternatively, some drugs increase the expression of CYP enzymes, resulting in a lower concentration of a drug normally metabolized by these enzymes. In these scenarios, the dose may need to be adjusted to achieve optimal efficacy and safety.
CDER clinical pharmacologists analyzing PBPK models that predict CYP enzyme–mediated DDIs have found that PBPK modeling can predict the effects of CYP enzyme inhibitors with confidence in certain cases. This approach has been used to predict changes in the concentrations of many victim drugs in the absence of a dedicated clinical DDI study. These predictions have been used to derive dosing recommendations and inform the labeling for these drugs (e.g., including dose adjustments for patients taking other medications in the Dosing and Administration section of the label). This approach has been successful with drugs such as cancer therapeutics and drugs to treat rare diseases. CDER clinical pharmacologists have also shown that in some cases, PBPK modeling can help rule out the potential for a significant DDI or eliminate the need for a clinical study even when the drug candidate itself is a perpetrator of the DDI.
DDIs may also occur through effects on various drug transporters, which are the proteins responsible for the uptake or flow of drugs across cellular membranes and into or out of various organs and tissues. CDER clinical pharmacologists are carefully assessing PBPK models of transporter-mediated drug interactions in regulatory submissions and have identified gaps in our knowledge that have thus far limited the application of PBPK models to these kinds of DDIs.
Leading the Integration of Modeling in Drug Development and Evaluation
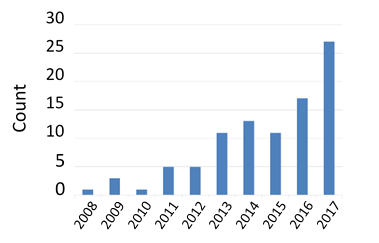
Figure 2. Number of NDAs per year containing analyses based on physiologically based pharmacokinetic modeling.
Over the past decade, collaborative efforts involving industry, academia, and regulatory scientists have led to a steady increase in the number of regulatory submissions that include PBPK models (Figure 2). As scientific understanding of the factors controlling drug disposition and action advances, CDER clinical pharmacologists anticipate a growing number of regulatory submissions that use modeling approaches.
CDER clinical pharmacologists recently published a guidance on how to report results of PBPK modeling and simulation studies in regulatory submissions. This document is intended to help standardize the content and format of these reports to facilitate FDA’s assessment of evidence from PBPK models. CDER pharmacologists are also continually engaging with drug developers through workshops, official meetings, and other forms of communication to ensure that the full potential of modeling is realized.
How does this research improve the drug development process?
Thanks to CDER research, the PBPK modeling approach for predicting enzyme-mediated DDI potential is being used extensively in lieu of clinical trials to help avoid unnecessary drug exposures, reduce development costs, and save time. CDER researchers are studying how to improve the reliability of PBPK model predictions in other areas of application, such as predicting pharmacokinetics in specific populations (e.g., children, the elderly, pregnant women, people with chronic diseases), assessing the potential for transporter-mediated DDIs, and drug product optimization.
Scientific Publications and Guidances Based on this Research
- Zhao, P, L Zhang, JA Grillo, Q Liu, JM Bullock, Moon YJ, et al, 2011, Applications of Physiologically Based Pharmacokinetic (PBPK) Modeling and Simulation During Regulatory Review, Clinical Pharmacology & Therapeutics, 89 (2): 259-267.
- Zhao, P, M Rowland, SM Huang, 2012, Best Practice in the Use of Physiologically Based Pharmacokinetic Modeling and Simulation to Address Clinical Pharmacology Regulatory Questions, Clinical Pharmacology & Therapeutics, 92 (1): 17-20.
- Vieira, MdLT, P Zhao, EG Berglund, KS Reynolds, L Zhang, LJ Lesko, SM Huang, 2012, Predicting Drug Interaction Potential With A Physiologically Based Pharmacokinetic Model: A Case Study Of Telithromycin, A Time-Dependent CYP3A Inhibitor, Clinical Pharmacology & Therapeutics, 91 (4): 700-708.
- Leong, R, MLT Vieira, P Zhao, Y Mulugeta, CS Lee CS, SM Huang, GJ Burckart, 2012, Regulatory Experience With Physiologically Based Pharmacokinetic Modeling for Pediatric Drug Trials, Clinical Pharmacology & Therapeutics, 91 (5): 926-931.
- Vieira, MdLT, MJ Kim MJ, S Apparaju S, V Sinha V, Zineh I, Huang SM, Zhao P, 2014, PBPK Model Describes the Effects of Co-Medication and Genetic Polymorphism on Systemic Exposure of Drugs that Undergo Multiple Clearance Pathways, Clinical Pharmacology & Therapeutics, 95 (5): 550-557.
- Sinha, V, P Zhao, SM Huang, I Zineh, 2014, Physiologically Based Pharmacokinetic Modeling: From Regulatory Science to Regulatory Policy, Clinical Pharmacology & Therapeutics, 95 (5): 478-480.
- Wagner, C, Y Pan, V Hsu, JA Grillo, L Zhang, KS Reynolds, et al., 2015, Predicting the Effect of Cytochrome p450 inhibitors on Substrate Drugs: Analysis of Physiologically Based Pharmacokinetic Modeling Submissions to the US Food and Drug Administration, Clinical Pharmacokinetics,54 (1): 117-127.
- Wagner C, Y Pan, V Hsu, JA Grillo, L Zhang, KS Reynolds, et al., 2016, Predicting the Effect of CYP3A Inducers on the Pharmacokinetics of Substrate Drugs Using Physiologically Based Pharmacokinetic (PBPK) Modeling: An Analysis of PBPK Submissions to the US FDA, Clinical Pharmacokinetics, 54 (1): 117-127.
- Wagner, C, P Zhao, Y Pan, V Hsu, J Grillo, SM Huang, V Sinha, 2015, Application of Physiologically Based Pharmacokinetic (PBPK) Modeling to Support Dose Selection: Report of an FDA Public Workshop on PBPK, CPT: Pharmacometrics & Systems Pharmacology, 4 (4), 226-230.
- Pan, Y, V Hsu, M Grimstein, L Zhang, V Arya, V Sinha, et al., 2016, The Application of Physiologically Based Pharmacokinetic Modeling to Predict the Role of Drug Transporters: Scientific and Regulatory Perspectives. Journal of Clinical Pharmacology, 56 (Suppl 7): S122-S131.
- Hsueh, CH, V Hsu, P Zhao, L Zhang, KM Giacomini, SM Huang, 2018, PBPK Modeling of the Effect of Reduced Kidney Function on the Pharmacokinetics of Drugs Excreted Renally by Organic Anion Transporters. Clinical Pharmacology & Therapeutics. 103 (3):485-492.
- Hsu V, MLT Vieira, PZhao, L Zhang, JH Zheng, A Nordmark, et al., 2014, Towards Quantitation of the Effects of Renal Impairment and Probenecid Inhibition on Kidney Uptake and Efflux Transporters, Using Physiologically Based Pharmacokinetic Modelling and Simulations, Clinical Pharmacokinetics, 53 (3): 283-293.
- Tan, ML, K Yoshida, P Zhao, L Zhang, TD Nolin, M Piquette-Miller, et al., 2018, Effect of Chronic Kidney Disease on Nonrenal Elimination Pathways: A Systematic Assessment of CYP1A2, CYP2C8, CYP2C9, CYP2C19, and OATP, Clinical Pharmacology & Therapeutics,103 (5): 854-867.
- Tan, ML, P Zhao, L Zhang, YF Ho, MVS Varma, S Neuhoff S, et al., Use of Physiologically Based Pharmacokinetic Modeling to Evaluate the Effect of Chronic Kidney Disease on the Disposition of Hepatic CYP2C8 and OATP1B Drug Substrates, Clinical Pharmacology & Therapeutics, 2018 Aug 3. doi: 10.1002/cpt.1205. [Epub ahead of print]
- Grimstein, M, Y Yang, X Zhang, J Grillo, SM Huang, I Zineh, Y Wang, Physiologically-Based Pharmacokinetic (PBPK) Modeling in Regulatory Science: An Update from the US Food and Drug Administration’s Office of Clinical Pharmacology. Journal of Pharmaceutical Sciences, 2018 Oct 29. doi: 10.1016/j.xphs.2018.10.033. [Epub ahead of print]
- Guidance for industry: Physiologically based pharmacokinetic analyses — format and content (2016)
- Guidance for industry: In vitro metabolism- and transporter-mediated drug-drug interaction studies (2017)
- Guidance for industry: Clinical drug interaction studies — study design, data analysis, and clinical implications (2017)