Impact Story: Addressing Concerns About the Quality of Generic Drugs for Treating Epilepsy
When concerns arose in the medical community that generic versions of drugs used to treat epilepsy were not as effective as the brand name versions, FDA conducted groundbreaking clinical trials to address this issue.
The Scientific Challenge
Overview and basics related to the Generic Drugs Program at FDA
In 2007, in response to long-standing concerns among some patients and physicians that generic versions of drugs to treat epilepsy can lead to loss of seizure control, the American Epilepsy Society (AES) took a position opposing the substitution of generic epilepsy drugs for patients without the consent of physicians and patients. However, no scientific studies comparing generic and brand name antiepileptic drugs had been conducted. Although they were meant to protect patients, the recommendations would likely raise costs and limit the availability of new generics.
FDA's Collaborative Research to Determine the Bioequivalence of Brand Name and Generic Antiepileptic Drugs in Patients
Since 2010, FDA has led a collaborative effort to address these concerns by conducting three pivotal clinical trials in patients with epilepsy: the Bioequivalence in Epilepsy Patients (BEEP) study ![]() and the Equivalence Among Antiepileptic Drug Generic and Brand Products in People With Epilepsy (EQUIGEN) multiple
and the Equivalence Among Antiepileptic Drug Generic and Brand Products in People With Epilepsy (EQUIGEN) multiple ![]() - and single-dose studies
- and single-dose studies ![]() . In designing and conducting these trials, FDA reached out to academic medical centers, the National Institute of Neurological Disorders and Stroke, and AES to bring together key expertise and perspectives.
. In designing and conducting these trials, FDA reached out to academic medical centers, the National Institute of Neurological Disorders and Stroke, and AES to bring together key expertise and perspectives.
The BEEP study compared lamotrigine with the brand name version, Lamictal. Investigators studied 38 high-risk patients (those who reported problems or more seizures when switched to the generic). The patients were repeatedly switched between brand name and generic versions to compare the bioequivalence (as indicated by area under the curve and maximum concentration achieved) and study within-patient variability. The study found the generic was bioequivalent to Lamictal and had similar within-patient variability, providing strong evidence that the two drugs could be used interchangeably. No significant differences in control of epileptic seizures were observed.
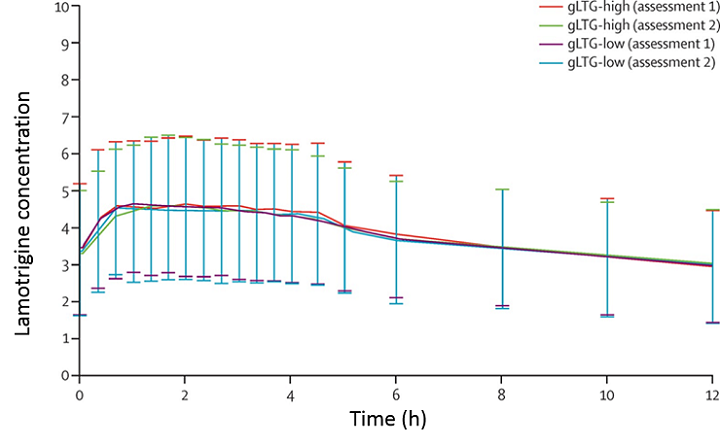
Figure 1. Read more about this figure
Next, FDA conducted the EQUIGEN multiple-dose study, a randomized trial comparing two generic versions of lamotrigine. The study compared the two generics that differed the most in terms of bioequivalence (one generic was at the high end and the other generic was at the low end of the bioequivalence spectrum) and inactive ingredients, as shown in Figure 1. As was the case in the BEEP study, the bioequivalence measures were very similar, and there were no significant differences in seizure control.
Even stronger evidence was provided by the EQUIGEN single-dose study, which compared the same two drugs used in the multiple-dose study with the brand name version in a single-dose trial. Single-dose trials are generally considered to be more sensitive to differences in formulation. As in the previous trials, there were no significant differences among the tested drugs.
FDA conducted all of these studies in close collaboration with the medical community, including professional organizations and medical researchers. The studies' results have strongly supported the bioequivalence approach to the evaluation of generic antiepileptic medications and prompted AES to modify its position on generic drug substitution for adults and children with epilepsy. In its 2016 position statement, AES said that the results of the bioequivalence studies confirmed the appropriateness of the FDA bioequivalence standards. AES recommended that descriptions of generic products for patients and caregivers "should indicate that generic products are equivalent to the brand product" and that for these patients, "counseling should not include descriptions of generic products as being a cheaper or lower-quality version of the brand product."
By providing strong clinical evidence supporting the equivalence of generic and brand name drugs to treat epilepsy, these studies help to allay concerns in the medical community about generic substitution and pave the way for lower costs to patients.
FDA Ensures the Quality of Extended-Release Epilepsy Drugs
Recently, FDA has also sought to ensure the quality of generics for extended-release formulations of drugs to treat epilepsy. Extended- or modified-release drugs are made by altering the physical characteristics of the tablet or capsule and the inactive ingredients (the substances other than the active ingredient, such as flavoring agents or colorants) so that the drug is released in a controlled way. Assurance about the quality of these drugs is especially important, because they may be taken just once a day, which can improve patient adherence and, thus, better seizure control.
One concern in the medical community involved reports that extended-release tablets of generic levetiracetam were appearing in the stools of epilepsy patients. FDA laboratory testing confirmed that the drug was completely released even if the tablets remained intact, and this information was added to the label to reassure physicians and patients.
In addition to conducting clinical and laboratory studies, FDA is addressing the differences between extended-release formulations of generic and brand name drugs by analyzing data in FDA's Adverse Event Reporting System (FAERS). FDA's Sentinel Initiative complements FAERS and supports active surveillance of information on the safety of medical products that is gathered from a variety of electronic data sources, including medical records. This new and continually expanding system will greatly expand FDA's capacity to quickly identify potential safety issues in both brand name and generic drugs of all kinds.
For more information, please visit the Office of Generic Drugs.
Related Publications
Privitera, MD, TE Welty, BE Gidal, et al., 2016, Generic-to-generic lamotrigine switches in people with epilepsy: The randomised controlled EQUIGEN trial ![]() , Lancet Neurol, 15:365—372, doi:10.1016/S1474-4422(16)00014-4.
, Lancet Neurol, 15:365—372, doi:10.1016/S1474-4422(16)00014-4.
Sun, D, H Wen, A Externbrink, et al., 2016, Ghost-Pill-Buster: A case study of intact Levetiracetam extended-release tablets after dissolution testing ![]() , CNS Drugs, 30:455—460, doi:10.1007/s40263-016-0332-9.
, CNS Drugs, 30:455—460, doi:10.1007/s40263-016-0332-9.
Ting, TY, W Jiang, R Lionberger, et al., 2015, Generic lamotrigine versus brand-name Lamictal bioequivalence in patients with epilepsy: A field test of the FDA bioequivalence standard ![]() , Epilepsia, 56:1415—1424, doi:10.1111/epi.13095.
, Epilepsia, 56:1415—1424, doi:10.1111/epi.13095.
Berg, M, TE Welty, BE Gidal, et al., 2017, Bioequivalence between generic and branded lamotrigine in people with epilepsy: The EQUIGEN randomized clinical trial ![]() , JAMA Neurol, Epub ahead of print, doi:10.1001/jamaneurol.2017.0497.
, JAMA Neurol, Epub ahead of print, doi:10.1001/jamaneurol.2017.0497.
Figure 1
Concerned that brand switching of generic antiepileptic drugs might lead to loss of seizure control, FDA conducted a clinical trial in which patients were assigned two different generic versions of lamotrigine (gLTG). The two drugs (referred to here as high or low) were chosen because they were the most disparate in terms of their bioequivalence results and other data. Switching between drugs in an individual patient (the sequence of treatment was either high, low, high, low or low, high, low, high) controlled for differences between patients. In terms of area under the curve — the extent of drug absorption — in each treatment period, the drugs were indistinguishable, and there was no loss of seizure control, indicating that physicians could confidently prescribe generic versions of lamotrigine. Learn more about this study ![]() .
.

