Learning from Patient Text Messaging to Optimize Opioid Prescribing and Reduce Misuse
CDER investigates the utility of a text messaging app to obtain patient-reported information that can help FDA improve opioid prescription guidelines for surgical patients.
The Public Health Challenge
The nonmedical use of physician-prescribed opioids is the second most common type of illicit drug use in the US. Opioid prescription rates for patients experiencing acute pain (e.g., those recovering from surgery) are especially high in the US, where it has been estimated that the median number of tablets prescribed for common surgical procedures is 20, compared with a median of one tablet in other countries, and that between 42% and 71% of opioids prescribed for acute pain remain unused. Such unused drugs may become available for misuse and abuse, perpetuating the opioid crisis. Although some state policies limit the number of opioids that can be prescribed for newly occurring pain, the regulations have been criticized as a one-size-fits-all approach that is disconnected from individual needs for pain relief. Research into the postoperative experiences of patients may help to improve prescribing guidelines for surgical procedures, but phone calls and other methods for surveying patient experiences are labor-intensive and are not easily scalable across the diverse contexts in which opioids are prescribed.
Improving information flow between prescribing physicians and patients
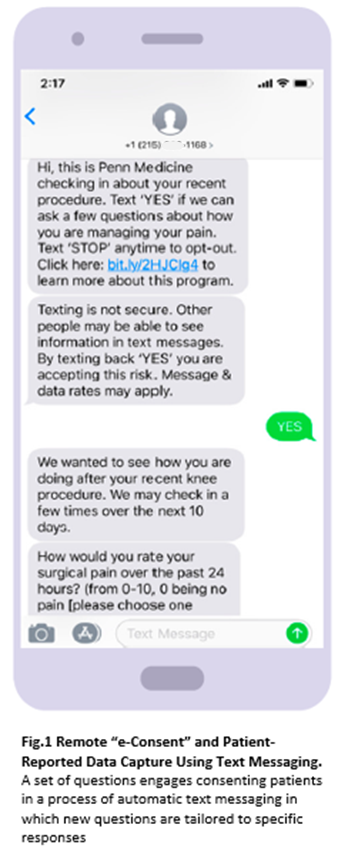
FDA’s Safe Use Initiative is committed to reducing preventable harm from drugs by soliciting and funding projects that develop innovative methods to create, facilitate, and encourage research in the area of safe medication use. Recognizing the need to draw from the patient perspectives and obtain data to properly balance pain management and responsible opioid stewardship, University of Pennsylvania researchers turned to the Safe Use Initiative to build, deploy, and test an automated text messaging program in conjunction with a secure platform. Their goal was to use software (provided by Mosio) that would enable patients to share information about their experiences and using prescribed opioids. By interacting with patients, initially via telephone and then via text messaging, the researchers were able, through an iterative process, to develop a set of logically connected messages that could be used to elicit key information from patients about opioid use, pain intensity, and pain manageability. The text messaging program was designed to simulate real conversation and to reliably translate patient responses (for example, when patient responded with slang expressions). An introductory message (Figure 1) familiarized patients with the study and obtained patient consent for additional messaging and data capture. If patients agreed to participate, a series of conversational questions was triggered to be sent as appropriate on postoperative days 4, 7, 14, and 21. In some situations (for example, when patients were unable to manage pain), automated triggers directing patients to their clinical team would be activated.
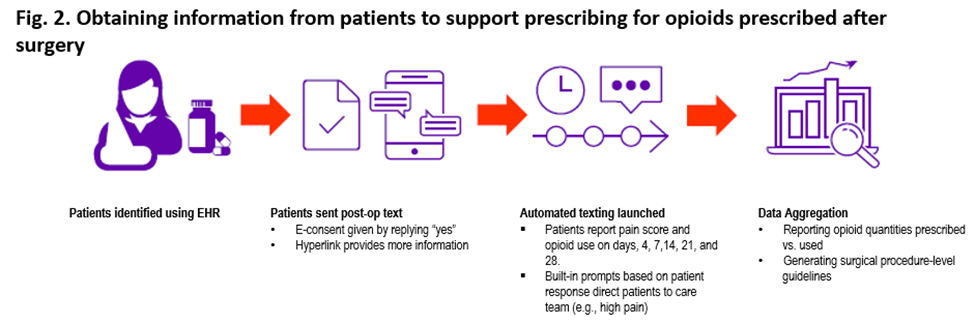
To test the value of this approach to gathering data to improve prescribing, the researchers conducted a study in 919 patients who had undergone urologic or orthopedic procedures and for whom the amount of opioids prescribed was available in their electronic health records (EHRs; see Figure 2).
The key findings of the investigation are listed below:
- Most opioid tablets were taken within the first four days after procedures, and patient ability to manage pain during this period was reportedly high, with individuals subsequently taking fewer or no opioid medications.
- Discordance in reports of pain intensity, ability to manage pain, and opioid use emerged early in the postoperative recovery period.
- During the study period, 15,581 tablets were prescribed and 9,452 tablets (60.7%) were left unused; most patients used less than half of their prescribed tablets.
The authors of the study offer their approach as 1) a real-time and continuous method for clinicians to gain understanding of postoperative pain and opioid use and 2) as a step toward translating patient-reported data into procedure-specific prescribing guidelines that can be built into clinical workflows. Ultimately, the goal of the research will be to guide shared decision-making so as to limit quantities of prescribed opioids. The research effort has benefited greatly from early collaboration with clinical providers and the ability to obtain the appropriate legal and IT support that has successfully driven enrollment, data capture, and analytics without unduly burdening clinical providers (Table 1).
| Organizational | Technical |
|---|---|
| Consensus building with key stakeholders | Collaboration with platforms in iterative design |
| Offloading clinical providers from consent processes | Clear messaging that can be dynamically updated |
| Focusing on privacy and security using digital methods | Continuous human quality assessment |
| Providing patient-reported data feedback to clinicians for quality improvement and guideline development | Integrated data organization and reporting |
How does this work improve the safety of prescribed opioids?
The automated text messaging program developed by CDER-funded researchers and collaborators provides an efficient way to understand patient needs for prescribed opioids after surgery; to ensure that patients in need have access to appropriate pain medications; and to prevent the prescription of excess quantities of drugs that could be diverted to misuse or abuse. This new approach to gathering needed information from patients may be useful for developing improved prescribing guidelines in diverse clinical contexts.
References
1. Agarwal AK, Lee D, Ali Z et al. Patient-Reported Opioid Consumption and Pain Intensity After Common Orthopedic and Urologic Surgical Procedures With Use of an Automated Text Messaging System. JAMA Netw Open. 2021 Mar 1;4(3):e213243. doi: 10.1001/jamanetworkopen.2021.3243. PMID: 33764425; PMCID: PMC7994954.
2. Agarwal, AK, Ali Z, Sennett B et al. An Automated Text Messaging Program to Inform Postoperative Opioid Prescribing. NEJM Catalyst no. 3 (2021).