Drug Trials Snapshots: POLIVY
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to POLIVY Prescribing Information for complete information.
POLIVY (polatuzumab vedotin-piiq)
poe li' vee
Genentech, Inc.
Approval date: June 10, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
POLIVY is used with other drugs to treat adults with diffuse large B-cell lymphoma (DLBCL) whose disease has come back or has not improved after at least two previous treatments.
DLBCL is a fast-growing cancer of the lymph system, which is part of the body’s immune system.
How is this drug used?
POLIVY is given by a healthcare provider directly into the bloodstream through a needle in the vein. This is known as an intravenous, or IV infusion. POLIVY is given every 21 days for 6 cycles together with bendamustine (a type of chemotherapy) and a rituximab product (a combination known as “BR”).
What are the benefits of this drug?
In a clinical trial, of 40 patients with DLBCL assigned to get POLIVY plus BR, 63% had complete or partial shrinkage of their tumors (remission), and 40% had complete shrinkage (complete remission) after finishing treatment.
In comparison, of 40 patients assigned to get BR alone, 25% had a remission, and 18% had a complete remission after finishing treatment.
POLIVY was approved under FDA’s accelerated approval program, which provides earlier patient access to a promising new drug while the company continues to conduct clinical trials to confirm that the drug works well.
There are ongoing clinical trials to confirm the clinical benefit of POLIVY.
What are the benefits of this drug (results of trials used to assess efficacy)?
Presented below are the response rates and duration of response (DOR) as assessed by an Independent Review Committee (IRC).
Table 2. Response Rates in Patients with Relapsed or Refractory DLBCL
|
Response per IRC, n (%)a |
POLIVY + BR |
BR |
|
n = 40 |
n = 40 |
|
|
Objective Response at End of Treatmentb (95% CI) |
18 (45) (29, 62) |
7 (18) (7, 33) |
|
CR (95% CI) |
16 (40) (25, 57) |
7 (18) (7, 33) |
|
Difference in CR rates, % (95% CI)c |
22 (3, 41) |
|
|
Best Overall Response of CR or PRd (95% CI) |
25 (63) (46, 77) |
10 (25) (13, 41) |
|
Best Response of CR (95% CI) |
20 (50) (34, 66) |
9 (23) (11, 38) |
BR= bendamustine and rituximab; CI = confidence interval; CR = complete response; PR = partial remission;
a PET-CT based response per modified Lugano 2014 criteria. Bone marrow confirmation of PET-CT CR was required. PET‑CT PR required meeting both PET criteria and CT criteria for PR.
b End of treatment was defined as 6–8 weeks after Day 1 of Cycle 6 or last study treatment.
c Miettinen-Nurminen method
d PET-CT results were prioritized over CT results.
|
POLIVY Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
The trial that looked at the benefit of POLIVY was too small to determine if there were any differences in sex, race and age subgroups.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The table below summarizes efficacy results by demographic subgroups. Because of the small sample sizes, these exploratory analyses should be interpreted with caution.
Table 3. Subgroup Analyses of CR
Demographic Subgroup |
POLIVY+BR |
BR |
|---|---|---|
|
Sex |
||
|
Women |
5/12, 42 (15, 72) |
3/15, 20 (4, 48) |
|
Men |
11/28, 39 (22,59) |
4/25, 16 (5,36) |
|
Race |
||
|
American Indian or Alaska Native |
NA |
0/1, 0 (0, 98) |
|
Asian |
3/6, 50 (12, 88) |
1/4, 25 (1, 81) |
|
Black or African American |
0/3, 0 (0, 71) |
NA |
|
Unknown |
2/5, 40 (5, 85) |
0/4, 0 (0, 60) |
|
White |
11/26, 42 (23, 63) |
6/31, 19 (7, 37) |
|
Age |
||
|
< 65 years |
6/17, 35 (14, 62) |
2/14, 14 (2, 43) |
|
>= 65 years |
10/23, 43 (23, 66) |
5/26, 19 (7, 39) |
|
Region |
||
|
Non-USA |
12/24, 50 (29, 71) |
5/32, 16 (5, 33) |
|
USA |
4/16, 25 (7, 52) |
2/8, 25 (3, 65) |
Adapted from FDA review
What are the possible side effects?
POLIVY may cause serious side effects including nerve damage (peripheral neuropathy), infusion related allergic reactions, bone marrow suppression, infections including a rare type of brain infection (progressive multifocal leukoencephalopathy), tumor lysis syndrome (caused by fast breakdown of cancer cells), liver damage, and harm to an unborn baby.
The most common side effects of POLIVY are low blood cell counts, nerve damage, fatigue, diarrhea, fever, decrease appetite, and pneumonia.
What are the possible side effects (results of trials used to assess safety)?
The tables below summarize adverse reactions and laboratory abnormalities occurring in the trial. Presented is a safety population which includes 39 patients per arm from the efficacy population and an additional 6 patients treated with POLIVY + BR in the same trial.
Table 4. Adverse Reactions Occurring in >10% of Patients with Relapsed or Refractory DLBCL and ≥5% more in the POLIVY Plus Bendamustine and Rituximab Product Group
|
Adverse Reactions by Body System |
POLIVY + BR n = 45 |
BR n = 39 |
||
|
All Grades, % |
Grade 3 or Higher, % |
All Grades, % |
Grade 3 or Higher, % |
|
|
Blood and Lymphatic System Disorders |
||||
|
Neutropenia |
49 |
42 |
44 |
36 |
|
Thrombocytopenia |
49 |
40 |
33 |
26 |
|
Anemia |
47 |
24 |
28 |
18 |
|
Lymphopenia |
13 |
13 |
8 |
8 |
|
Nervous System Disorders |
||||
|
Peripheral neuropathy |
40 |
0 |
8 |
0 |
|
Dizziness |
13 |
0 |
8 |
0 |
|
Gastrointestinal Disorders |
||||
|
Diarrhea |
38 |
4.4 |
28 |
5 |
|
Vomiting |
18 |
2.2 |
13 |
0 |
|
General Disorders |
||||
|
Infusion-related reaction |
18 |
2.2 |
8 |
0 |
|
Pyrexia |
33 |
2.2 |
23 |
0 |
|
Decreased appetite |
27 |
2.2 |
21 |
0 |
|
Infections |
||||
|
Pneumonia |
22 |
16 a |
15 |
2.6 b |
|
Upper respiratory tract infection |
13 |
0 |
8 |
0 |
|
Investigations |
|
|
|
|
|
Weight decreased |
16 |
2.2 |
8 |
2.6 |
|
Metabolism and Nutrition Disorders |
||||
|
Hypokalemia |
16 |
9 |
10 |
2.6 |
|
Hypoalbuminemia |
13 |
2.2 |
8 |
0 |
|
Hypocalcemia |
11 |
2.2 |
5 |
0 |
The table includes a combination of grouped and ungrouped terms. Events were graded using NCI
CTCAE version 4.
a Includes 2 events with fatal outcome.
b Includes 1 event with fatal outcome.
Table 5. Selected Laboratory Abnormalities Worsening from Baseline in Patients with Relapsed or Refractory DLBCL and ≥5% more in the POLIVY Plus Bendamustine and Rituximab Product Group
|
Laboratory Parameter a |
POLIVY + BR |
BR |
||
|
n = 45 |
n = 39 |
|||
|
All Grades, (%) |
Grade 3–4, (%) |
All Grades, (%) |
Grade 3–4, (%) |
|
|
Hematologic |
||||
|
Lymphocyte count decreased |
87 |
87 |
90 |
82 |
|
Neutrophil count decreased |
78 |
61 |
56 |
33 |
|
Hemoglobin decreased |
78 |
18 |
62 |
10 |
|
Platelet count decreased |
76 |
31 |
64 |
26 |
|
Chemistry |
||||
|
Creatinine increased |
87 |
4.4 |
77 |
5 |
|
Albumin decreased |
47 |
4.4 |
38 |
2.6 |
|
Calcium decreased |
44 |
9 |
26 |
0 |
|
SGPT/ALT increased |
38 |
0 |
8 |
2.6 |
|
SGOT/AST increased |
36 |
0 |
26 |
2.6 |
|
Lipase increased |
36 |
9 |
13 |
5 |
|
Phosphorus decreased |
33 |
7 |
28 |
8 |
|
Amylase increased |
24 |
0 |
18 |
2.6 |
|
Potassium decreased |
24 |
11 |
28 |
5 |
aIncludes laboratory abnormalities that are new or worsening in grade or with worsening from baseline unknown.
POLIVY Prescribing Information
Were there any differences in side effects among sex, race and age?
The trial that looked at the side effects of POLIVY was too small to determine if there were any differences in sex, race and age subgroups.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The tables below summarize selected adverse reactions by sex and age subgroup in safety population. Because of the small sample sizes, these exploratory analyses should be interpreted with caution.
Table 6. Subgroup Analyses of Adverse Reactions by Sex
|
Adverse Reaction |
Women |
Men |
||
|
POLIVY+BR |
BR |
POLIVY+BR |
BR |
|
|
Serious Adverse Reaction |
100 |
64 |
81 |
76 |
|
Peripheral Neuropathy |
43 |
7 |
39 |
8 |
Trial data
Table 7. Subgroup Analyses of Adverse Reactions by Age Group
|
Adverse Reaction |
< 65 Years of Age
|
≥ 65 Years of Age |
||
|
POLIVY+BR |
BR |
POLIVY+BR |
BR
|
|
|
Serious Adverse Reaction |
68 |
46 |
65 |
69 |
|
Peripheral Neuropathy |
32 |
0 |
46 |
12 |
Trial data
| In an expanded safety population (N=173) which included all patients who received at least one dose of POLIVY in this trial, patients aged ≥ 65 years had a numerically higher incidence of serious adverse reactions (61/95 or 64%) than patients aged < 65 years (41/78 or 53%). |
FDA review
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved POLIVY based primarily on evidence from one clinical trial (NCT02257567) that was conducted in the United States, Canada, Europe, and Asia. Patients who participated in the trial had lymphoma that came back or did not improve after prior treatment.
Presented below is the trial population that provided data to assess the benefit of POLIVY (called the efficacy population).
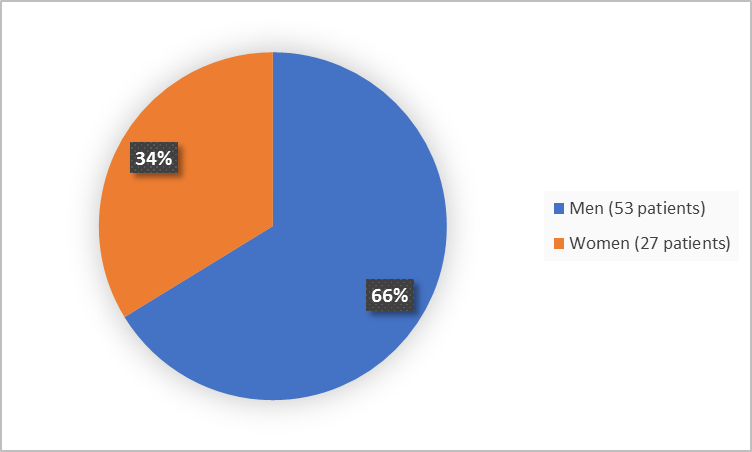
Figure 1 summarizes how many men and women participated in the trial (efficacy population).
Figure 1. Baseline Demographics by Sex (Efficacy Population)
FDA Review
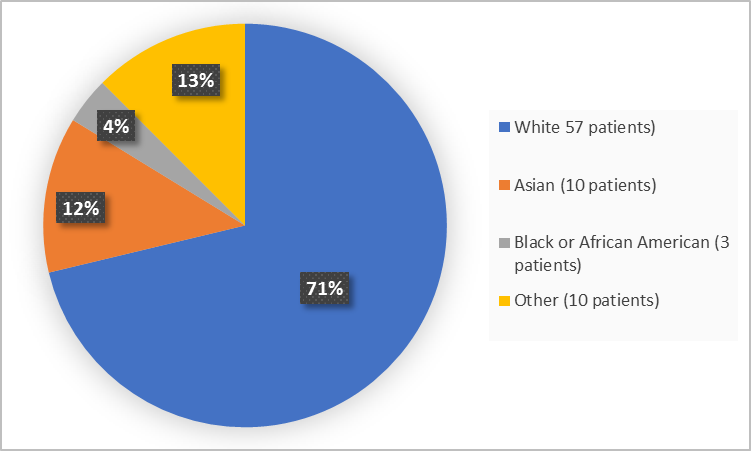
Figure 2 and Table 1 below summarize patients by race in the trial.
Figure 2. Baseline Demographics by Race (Efficacy Population)
FDA Review
Table 1. Baseline Demographics by Race (Efficacy Population)
|
Race |
Number of Patients |
Percentage |
|
White |
57 |
71 |
|
Black or African American |
3 |
4 |
|
Asian |
10 |
13 |
|
American Indian or Alaska Native |
1 |
1 |
|
Unknown |
9 |
11 |
FDA review
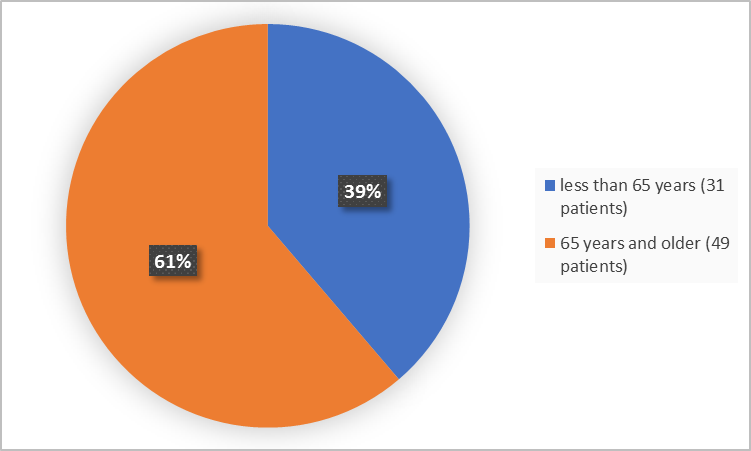
Figure 3. Baseline Demographics by Age (Efficacy Population)
FDA Review
The table below summarizes trial demographics.
Table 8. Baseline Demographics of Patients (Efficacy Population)
Demographic Parameter |
POLIVY+BR |
BR |
Overall |
|
Sex, n (%) |
|||
|
Women |
12 (30) |
15 (38) |
27 (34) |
|
Men |
28 (70) |
25 (62) |
53 (66) |
|
Race, n (%) |
|||
|
American Indian or Alaska Native |
0 (0) |
1 (2) |
1 (1) |
|
Asian |
6 (15) |
4 (10) |
10 (12) |
|
Black or African American |
3 (8) |
0 (0) |
3 (4) |
|
Unknown |
5 (12) |
4 (10) |
9 (11) |
|
White |
26 (65) |
31 (78) |
57 (71) |
|
Age group, n (%) |
|||
|
< 65 years |
17 (42) |
14 (35) |
31 (39) |
|
≥ 65 years |
23 (57) |
26 (65) |
49 (61) |
|
Ethnicity |
|||
|
Hispanic or Latino |
1 (3) |
1 (3) |
2 (3) |
|
Not Hispanic or Latino |
35 (88) |
36 (90) |
71 (89) |
|
Unknown |
4 (10) |
3 (8) |
7 (9) |
|
Region, n (%) |
|||
|
Asia/Pacific |
7 (18) |
6 (15) |
13 (16) |
|
Eastern Europe |
9 (22) |
11 (28) |
20 (25) |
|
North America |
17 (42) |
11 (28) |
28 (35) |
|
United States |
16 (40) |
8 (20) |
24 (30) |
|
Western Europe |
7 (18) |
12 (30) |
19 (24) |
Adapted from FDA review
How were the trials designed?
There was one trial that evaluated the benefit and side effects of POLIVY in patients with lymphoma whose disease has returned or has not improved after previous treatment.
Patients received either POLIVY in combination with bendamustine and rituximab or bendamustine and rituximab only every 21 days for 6 treatment cycles. Both patients and health care providers knew which treatment had been given.
The benefit of POLIVY was evaluated by measuring how many patients treated with POLIVY in combination with bendamustine and rituximab had a remission (complete or partial tumor shrinkage) in comparison to patients treated with bendamustine and rituximab only, and how long that response lasted.
How were the trials designed?
The safety and efficacy of POLIVY were established in an open label, multicenter trial in adult patients with relapsed or refractory lymphoma. The trial included a randomized portion that compared POLIVY in combination with BR to BR alone for six 21-day cycles in patients with DLBCL.
Efficacy was based on CR rate at the end of treatment and DOR, as determined by an IRC. Other efficacy measures included IRC-assessed best overall response.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION