Drug Trial Snapshot: TEPEZZA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the TEPEZZA Package Insert for complete information.
TEPEZZA (teprotumumab-trbw)
(tep-ez-zə)
Horizon
Approval date: January 21, 2020
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
TEPEZZA is a drug for the treatment of thyroid eye disease.
Thyroid eye disease is a rare, autoimmune disease characterized by proptosis (a condition where the eyes are pushed forward and bulge outward) leading to eye pain, double vision and difficulty closing the eyelid.
How is this drug used?
TEPEZZA is an injection. It is given by a healthcare provider directly into the vein (an intravenous infusion) once every three weeks for a total of eight infusions. It takes about 60-90 minutes to receive the infusion.
What are the benefits of this drug?
After 6 months of treatment, a higher proportion of patients treated with TEPEZZA (71-83%) demonstrated reduction in proptosis in comparison to patients treated with placebo (10-20%).
What are the benefits of this drug (results of trials used to assess efficacy)?
Table 1 shows efficacy results for individual trials. The proptosis responder rate at week 24 was defined as the percentage of patients with ≥2 mm reduction in proptosis in the study eye from baseline, without deterioration in the non-study eye (≥2mm increase).
Table 1. Efficacy Results in Patients with Thyroid Eye Disease in Trials 1 and 2
|
|
Trial 1 |
Trial 2 |
||||
|---|---|---|---|---|---|---|
|
TEPEZZA |
Placebo |
Difference |
TEPEZZA |
Placebo |
Difference |
|
|
Proptosis responder rate at week 24, |
71% (30) |
20% (9) |
51% |
83% (34) |
10% (4) |
73% |
|
Proptosis (mm) average change from baseline through week 24, |
-2.5 (0.2) |
-0.2 |
-2.3 |
-2.8 (0.2) |
-0.5 |
-2.3 |
1 Difference and its corresponding 95% Confidence Interval (CI) is based on a weighted average of the difference within each randomization stratum (tobacco user, tobacco non-use) using CMH weights.
2 Results were obtained from an MMRM with an unstructured covariance matrix and including treatment, smoking status, baseline value, visit, treatment by visit, and visit by baseline value interaction as fixed effects. A change from Baseline of 0 was imputed at the first post-Baseline visit for any subject without a post-Baseline value.
TEPEZZA Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: TEPEZZA worked similarly in men and women.
- Race: The majority of patients were White. The number of patients in other races were limited; therefore, differences in how TEPEZZA worked among races could not be determined.
- Age: TEPEZZA worked similarly in patients above and below 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age?
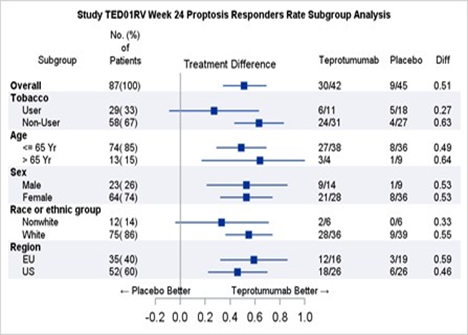
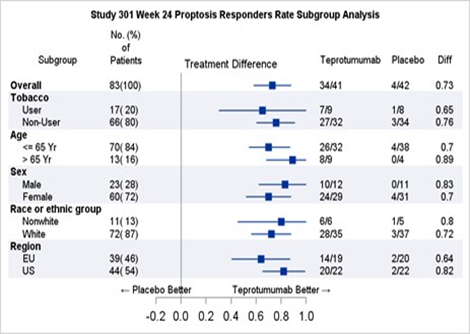
Table 2 shows the efficacy results by relevant demographic subgroups.
Table 2. Proptosis Response Subgroup Efficacy Analyses
Teprotumumab=TEPEZZA
CI=confidence interval
Adapted from FDA Review
What are the possible side effects?
TEPEZZA may cause serious side effects including infusion reactions, worsening of inflammatory bowel disease, and high blood sugar.
The most common side effects of TEPEZZA are muscle spasm, nausea, hair loss, diarrhea, fatigue, high blood sugar, dry skin, change in taste, and headache.
What are the possible side effects (results of trials used to assess safety)?
Table 3 below summarizes common adverse reactions from Trials 1 and 2.
Table 3. Adverse Reactions Occurring in 5% or More of Patients Treated with TEPEZZA and Greater Incidence than Placebo
|
Adverse Reactions |
TEPEZZA |
Placebo |
|---|---|---|
|
Muscle spasms |
21 (25%) |
6 (7%) |
|
Nausea |
14 (17%) |
8 (9%) |
|
Alopecia |
11 (13%) |
7 (8%) |
|
Diarrhea |
10 (12%) |
7 (8%) |
|
Fatiguea |
10 (12%) |
6 (7%) |
|
Hyperglycemiab |
8 (10%) |
1 (1%) |
|
Hearing impairmentc |
8 (10) |
0 |
|
Dysgeusia |
7 (8%) |
0 |
|
Headache |
7 (8%) |
6 (7%) |
|
Dry skin |
7 (8%) |
0 |
aFatigue includes asthenia
bHyperglycemia includes blood glucose increase
cHearing impairment (includes deafness, eustachian tube dysfunction, hyperacusis, hypoacusis and autophony)
TEPEZZA Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: Occurrence of side effects was similar in men and women.
- Race: The majority of patients were White. The number of patients in other races were limited; therefore, differences in side effects among races could not be determined.
- Age: The occurrence of side effects was similar in patients above and below 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Analyses of side effects by subgroups are presented below.
Table 4. Summary of Adverse Events by Sex
|
|
Men
|
Women
|
||
|---|---|---|---|---|
|
TEPEZZA |
Placebo |
TEPEZZA |
Placebo |
|
|
Any TEAE, n (%) |
19 (70.4) |
14 (73.7) |
48 (84.2) |
46 (68.7) |
|
Muscle spasms, n (%) |
5 (18.5) |
0 |
16 (28.1) |
6 (9) |
|
Nausea, n (%) |
4 (14.8) |
0 |
10 (17.5) |
8 (11.9) |
Table 5. Summary of Adverse Events by Race
|
|
White
|
Black or African American
|
||
|---|---|---|---|---|
|
TEPEZZA N=72 |
Placebo N=75 |
TEPEZZA N=8 |
Placebo N=6 |
|
|
Any TEAE, n (%) |
58 (80.6) |
51 (68) |
6 (75) |
4 (75) |
|
Muscle spasms, n (%) |
19 (26.4) |
5 (6.7) |
0 |
0 |
|
Nausea, n (%) |
11 (15.3) |
6 (8) |
1 (12.5) |
1 (16.7) |
Table 6. Summary of Adverse Events by Age
|
|
< 65 years
|
≥65 years
|
||
|---|---|---|---|---|
|
TEPEZZA N=71 |
Placebo N=73 |
TEPEZZA N=13 |
Placebo N=13 |
|
|
Any TEAE, n (%) |
56 (78.9) |
50 (68.5) |
11 (84.6) |
10 (76.9) |
|
Muscle spasms, n (%) |
20 (28.2) |
4 (5.5) |
1 (7.7) |
2 (15.4) |
|
Nausea, n (%) |
13 (18.3) |
7 (9.6) |
1 (7.7) |
1 (7.7) |
TEAE=treatment -emergent adverse event
Clinical Trial Report
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved TEPEZZA based on evidence from two clinical trials (Trial 1/ NCT01868997 and Trial 2/ NCT03298867) of 170 patients with active thyroid eye disease. The trials were conducted at 28 sites in Europe and United States.
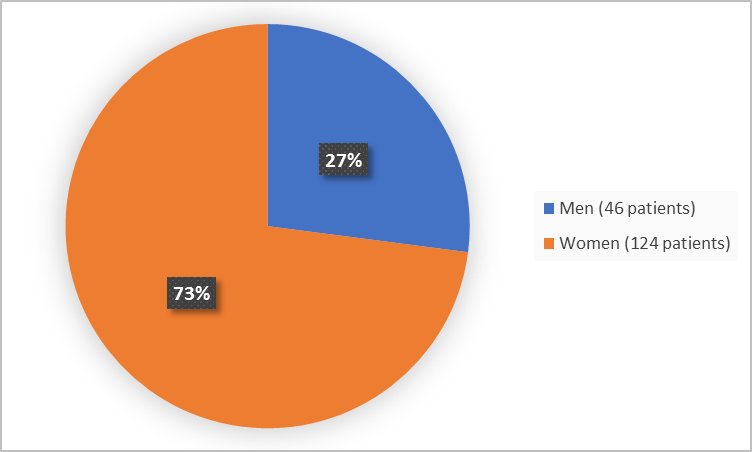
Figure 1 summarizes by sex how many patients were in the combined clinical trials.
Figure 1. Baseline Demographics by Sex
Adapted from FDA Review
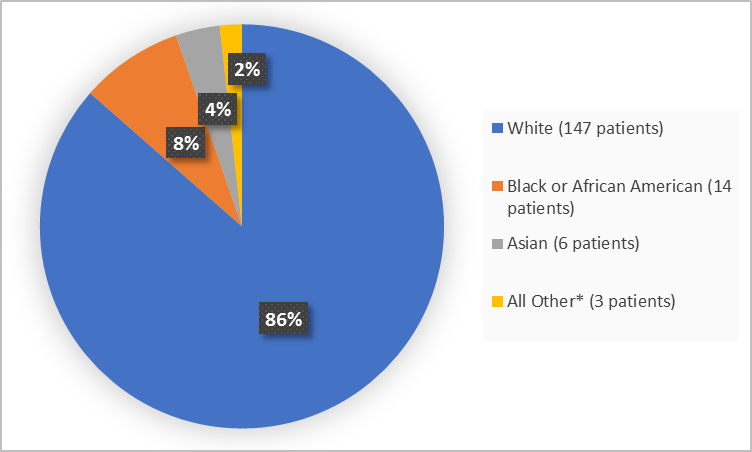
Figure 2 summarizes patients by race in the clinical trials.
Figure 2. Baseline Demographics by Race
*includes Native Hawaiian or other Pacific Islander and Other
Adapted from FDA Review
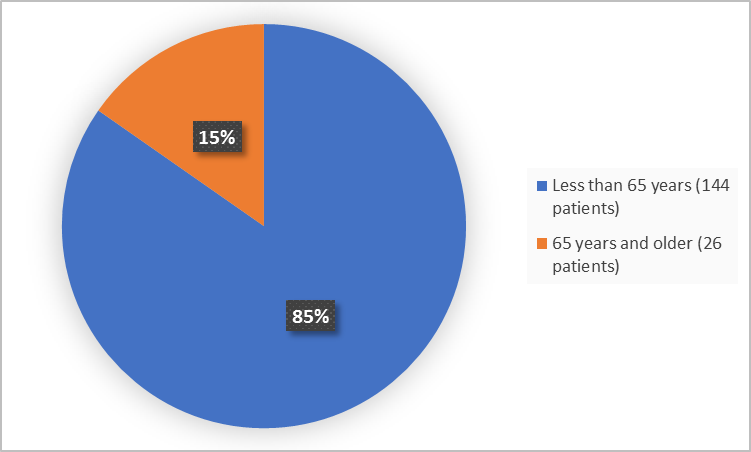
Figure 3 summarizes patients by age in the clinical trials.
Figure 3. Baseline Demographics by Age
Adapted from FDA Review
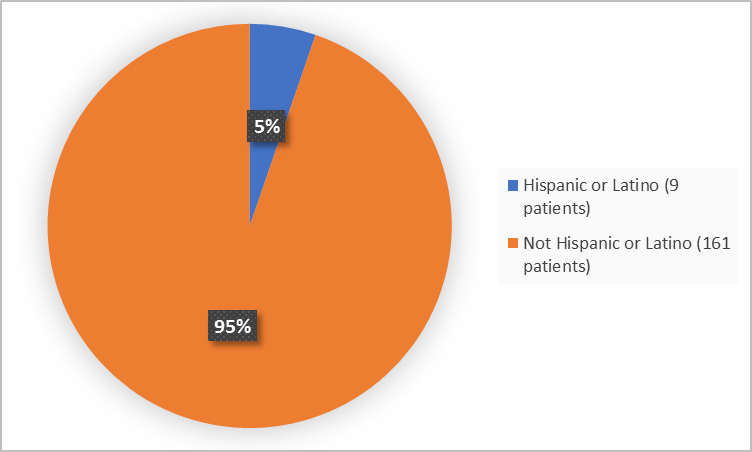
Figure 4 summarizes patients by ethnicity in the clinical trials.
Figure 4. Baseline Demographics by Ethnicity
Adapted from FDA Review
Who participated in the trials?
The demographic characteristics of population are summarized in Table 7.
Table 7. Demographic Characteristics
|
Demographic Category |
TEPEZZA n(%) |
Placebo N=86 n(%) |
Total N=170 n(%) |
|---|---|---|---|
|
Sex |
|||
|
Men |
27 (32.1) |
19 (22.1) |
46 (27.1) |
|
Women |
57 (67.9) |
67 (77.9) |
124 (72.9) |
|
Race |
|||
|
White |
72 (85.7) |
75 (87.2) |
147 (86.5) |
|
Black or African American |
8 (9.5) |
6 (7) |
14 (8.2) |
|
Asian |
3 (3.6) |
3 (3.5) |
6 (3.5) |
|
Native Hawaiian or Other Pacific Islander |
1 (1.2) |
0 |
1 (0.6) |
|
Other |
0 |
2 (2.3) |
2 (1.2) |
|
Age (years) |
|||
|
Median |
50.5 |
53 |
52 |
|
Min, Max |
22,79 |
20,77 |
20,79 |
|
Age Group |
|||
|
<65 years |
71 (84.5) |
73 (84.9) |
144 (84.7) |
|
≥65 years |
13 (15.5) |
13 (15.1) |
26 (15.3) |
|
Ethnicity |
|||
|
Hispanic or Latino |
4 (4.8) |
5 (5.8)) |
9 (5.3) |
|
Not Hispanic or Latino |
80 (95.2) |
81 (94.2) |
161 (94.7) |
|
Region |
|||
|
USA |
49 (58.3) |
47 (54.6) |
96 (56.5) |
|
Europe |
35 (41.7) |
39 (45.3) |
74 (43.5) |
Adapted from FDA Review
How was the trial designed?
There were two trials that provided data for TEPEZZA approval. Both trials enrolled patients with active thyroid eye disease.
Patients received TEPEZZA or placebo by intravenous infusion every three weeks for a total of 8 infusions.
After 24 weeks, the trials compared the percentage of patients who achieved greater than 2 mm reduction in proptosis between TEPEZZA and placebo.
How were the trials designed?
There were two randomized, double-masked, placebo-controlled trials that provided data for approval of TEPEZZA.
Patients with active thyroid eye disease received TEPEZZA by intravenous infusions (10 mg/kg for first infusion and 20 mg/kg for the remaining 7 infusions) or placebo infusions every 3 weeks for a total of 8 infusions.
The main outcome measure was the proptosis responder rate at week 24 defined as the percentage of patients with ≥2 mm reduction in proptosis in the study eye from baseline, without deterioration in the non-study eye (≥2 mm increase).
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION