Drug Trial Snapshot: AYVAKIT
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the AYVAKIT Package Insert for complete information.
AYVAKIT (avapritinib)
(aye' vah kit)
Blueprint Medicines Corporation
Approval date: January 9, 2020
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
AYVAKIT is used to treat adult patients with gastrointestinal stromal tumor (GIST) whose disease:
- is caused by certain abnormal platelet-derived growth factor receptor alpha (PDGFRA) genes and,
- cannot be surgically removed or,
- has spread throughout the body (metastatic GIST).
GIST is type of stomach, bowel, or esophagus tumor.
How is this drug used?
AYVAKIT is a tablet taken once daily on an empty stomach.
What are the benefits of this drug?
Thirty-six out of 43 patients (84%) experienced complete or partial shrinkage of the tumor which lasted six months or longer in 22 patients (61%).
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes efficacy results of the trial based on overall response rate (ORR) assessment by independent radiological review using modified RECIST v1.1criteria.
Table 1. Efficacy Results for Patients with GIST Harboring PDGFRA exon 18 Mutations, Including the Subgroup of Patients with PDGFR D842V Mutations in Trial 1
|
Efficacy Parameter |
PDGFRA exon 181 |
PDGFRA D842V |
|---|---|---|
|
Overall Response Rate (95% CI) |
84% (69%, 93%) |
89% (75%, 97%) |
|
Complete Response, n (%) |
3 (7%) |
3 (8%) |
|
Partial Response, n (%) |
33 (77%) |
31 (82%) |
|
Duration of Response |
n=36 |
n=34 |
|
Median in months (range) |
NR (1.9+, 20.3+) |
NR (1.9+, 20.3+) |
|
Patients with DOR ≥ 6-months, n (%)* |
22 (61%) |
20 (59%) |
CI=confidence interval; NR=not reached; NE=not estimable
+ Denotes ongoing response
1 Exon 18 mutations other than D842V included in this population are: deletion of D842_H845 (n=3); D842Y (n=1); and deletion of D842_H845 with insertion of V (n=1).
* 11 patients with an ongoing response were followed < 6 months from onset of response.
AYVAKIT Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: AYVAKIT worked similarly between men and women.
- Race: The majority of patients in the clinical trial were White. Differences among races could not be determined because of the small number of patients of other races.
- Age: AYVAKIT worked similarly in patients younger and older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The table below summarizes efficacy results based on overall response rate (ORR) by sex, race and age. Because of the small sample size, the exploratory analyses should be interpreted with caution.
Table 2. Efficacy Analyses by Subgroups
|
Demographic Group |
PDGFRα Exon 18 |
PDGFRα D842V |
||
|---|---|---|---|---|
|
|
ORR |
95% CI |
ORR |
95% CI |
|
Sex |
||||
|
Men |
23/29 (79) |
79,92 |
21/25 (84 |
64, 95 |
|
Women |
13/14 (93) |
66, 100 |
13/13 (100) |
75, 100 |
|
Race |
||||
|
White |
23/29 (79) |
60,92 |
22/25 (88) |
69, 97 |
|
Asian |
6/6 (100) |
54, 100 |
6/6 (100) |
54, 100 |
|
Black or African American |
2/3 (67) |
9, 99 |
2/3 (67) |
9, 99 |
|
Other/Unknown |
5/5 (100) |
48, 100 |
4/4 (100) |
40, 100 |
|
Age |
||||
|
<65 years |
21/24 (88) |
68, 97 |
20/22 (91) |
71, 99 |
|
≥65 years |
15/19 (79) |
54,94 |
14/16 (88) |
62, 98 |
|
Region |
||||
|
US |
9/13 (69) |
39,91 |
8/11 (73) |
39, 94 |
|
Europe |
22/25 (88) |
69,97 |
21/22 (95) |
77, 100 |
|
Asia |
5/5 (100) |
48,100 |
5/5 (100) |
48, 100 |
CI=confidence interval
FDA Review
What are the possible side effects?
AYVAKIT can cause serious side effects including bleeding inside the skull, effects on the central nervous system (e.g. forgetfulness, confusion, trouble thinking, change in mood or behavior etc.) and harm to a newborn baby.
The most common side effects of AYVAKIT are swelling, nausea, tiredness, trouble thinking, vomiting, decreased appetite, diarrhea, hair color change, increased tear secretion, abdominal pain, constipation, rash and dizziness.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions in the clinical trial. Presented is the safety population which includes all patients who received at least one dose of AYVAKIT (300 mg or 400 mg) orally once daily.
Table 3. Adverse Reactions (≥ 10%) in Patients Receiving AYVAKIT in Trial 1
|
Adverse Reactions |
AYVAKIT |
|
|---|---|---|
|
All Grades |
Grade ≥3 |
|
|
General |
||
|
Edemaa |
72 |
2 |
|
Fatigue/asthenia |
61 |
9 |
|
Pyrexia |
14 |
0.5 |
|
Gastrointestinal |
||
|
Nausea |
64 |
2.5 |
|
Vomiting |
38 |
2 |
|
Diarrhea |
37 |
4.9 |
|
Abdominal painb |
31 |
6 |
|
Constipation |
23 |
1.5 |
|
Dyspepsia |
16 |
0 |
|
Nervous System |
||
|
Cognitive impairmentc |
48 |
4.9 |
|
Dizziness |
22 |
0.5 |
|
Headache |
17 |
0.5 |
|
Sleep disordersd |
16 |
0 |
|
Taste effectse |
15 |
0 |
|
Mood disordersf |
13 |
1 |
|
Metabolism and nutrition |
||
|
Decreased appetite |
38 |
2.9 |
|
Eye |
||
|
Increased lacrimation |
33 |
0 |
|
Skin and subcutaneous tissue |
||
|
Rashg |
23 |
2.1 |
|
Hair color changes |
21 |
0.5 |
|
Alopecia |
13 |
- |
|
Respiratory, thoracic and mediastinal |
||
|
Dyspnea |
17 |
2.5 |
|
Pleural effusion |
12 |
2 |
|
Investigations |
||
|
Weight decreased |
13 |
1 |
*Per National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 and 5.0
a Edema includes face swelling, conjunctival edema, eye edema, eyelid edema, orbital edema, periorbital edema, face edema, mouth edema, pharyngeal edema, peripheral edema, edema, generalized edema, localized edema, peripheral swelling, testicular edema.
b Abdominal pain includes abdominal pain, upper abdominal pain, abdominal discomfort, lower abdominal pain, abdominal tenderness, and epigastric discomfort.
c Cognitive impairment includes memory impairment, cognitive disorder, confusional state, disturbance in attention, amnesia, mental impairment, mental status changes, encephalopathy, dementia, abnormal thinking, mental disorder, and retrograde amnesia.
d Sleep disorders includes insomnia, somnolence, and sleep
AYVAKIT Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: The majority of patients in the clinical trial were White. Differences in side effects among races could not be determined because of the small number of patients of other races.
- Age: The occurrence of side effects was similar in patients younger and older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The tables below summarize adverse reactions (ARs) during the clinical trial by sex, race and age subgroups.
Table 4. Occurrence of ARs by Sex Groups
|
System Organ Class |
Safety Population |
|
|---|---|---|
|
Women |
Men |
|
|
General disorders and administration site conditions |
||
|
Edemaa |
57 (71) |
88 (71) |
|
Fatigue/asthenia |
47 (59) |
77 (62) |
|
Pyrexia |
11 (14) |
18 (14) |
|
Gastrointestinal disorders |
||
|
Nausea |
51 (64) |
80 (65) |
|
Vomiting |
33 (41) |
45 (36) |
|
Diarrhea |
30 (38) |
45 (36) |
|
Abdominal painb |
29 (37) |
34 (26) |
|
Constipation |
19 (24) |
28 (23) |
|
Nervous system disorders |
||
|
Cogntive impairmentc |
37 (46) |
62 (50) |
|
Dizziness |
20 (25) |
25 (20) |
|
Headache |
18 (23) |
17 (14) |
|
Taste effectd |
11 (14) |
20 (16) |
|
Metabolism and nutritional disorders |
||
|
Decreased appetite |
31 (39) |
47 (38) |
|
Eye disorders |
||
|
Increased lacrimation |
26 (33) |
41 (33) |
|
Skin and subcutaneous tissue disorders |
||
|
Rashe |
22 (28) |
25 (20) |
|
Hair color changes |
17 (21) |
26 (21) |
|
Alopecia |
12 (15) |
15 (15) |
|
Respiratory, thoracic, and mediastinal disorders |
||
|
Dyspnea |
15 (19) |
20 (16) |
|
Pleural effusion |
12 (15) |
13 (10) |
aIncludes PT terms face swelling, conjunctival edema, eye edema, eyelid edema, orbital edema, periorbital edema, face edema, mouth edema, pharyngeal edema, peripheral edema, edema, generalized edema, localized edema, peripheral swelling, testicular edema.
b Includes PT terms of abdominal pain, upper abdominal, lower abdominal pain, abdominal discomfort, abdominal tenderness
cIncludes PT terms memory impairment, cognitive disorder, confusional state, disturbance in attention, mental impairment, somnolence, encephalopathy, mental status changes, disorientation, and abnormal thinking.
dIncludes PT terms dysgeusia and ageusia
eIncludes PT terms rash, rash maculo-papular, rash erythematous, rash macular, rash generalized, and rash papular
Table 5. Occurrence of ARs by Racial Groups
|
Preferred Term |
Safety Population |
||
|---|---|---|---|
|
White |
Other |
Unknown |
|
|
Fatigue |
85 (58) |
11 (31) |
17 (77) |
|
Periorbital edema |
64 (44) |
11 (31) |
7 (36) |
|
Decreased Appetite |
60 (41) |
7 (19) |
10 (45) |
|
Vomiting |
58 (40) |
9 (25) |
11 (50) |
|
Diarrhea |
57 (39) |
11 (31) |
8 (36) |
|
Increased lacrimation |
53 (36) |
5 (14) |
9 (41) |
|
Peripheral edema |
49 (34) |
7 (19) |
7 (32) |
|
Abdominal pain |
27 (18) |
10 (27) |
5 (23) |
Table 6. Occurrence of ARs by Age Groups
|
System Organ Class |
Safety Population |
|
|---|---|---|
|
<65 years |
≥65 years |
|
|
General disorders and administration site conditions |
||
|
Edemaa |
85 (70) |
62 (76) |
|
Fatigue/asthenia |
67 (55) |
57 (70) |
|
Pyrexia |
15 (12) |
14 (17) |
|
Gastrointestinal disorders |
||
|
Nausea |
79 (65) |
52 (63) |
|
Vomiting |
46 (38) |
32 (39) |
|
Diarrhea |
38 (31) |
37 (45) |
|
Abdominal painb |
|
|
|
Constipation |
26 (21) |
21 (26) |
|
Nervous system disorders |
||
|
Cogntive impairmentc |
57 (47) |
42 (51) |
|
Dizziness |
26 (21) |
19 (23) |
|
Headache |
24 (20) |
11 (13) |
|
Metabolism and nutritional disorders |
||
|
Decreased appetite |
41 (34) |
32 (45) |
|
Eye disorders |
||
|
Increased lacrimation |
32 (26) |
35 (43) |
|
Skin and subcutaneous tissue disorders |
||
|
Rashd |
31 (25) |
13 (16) |
|
Hair color changes |
31 (25) |
12 (15) |
|
Alopecia |
16 (13) |
11 (9) |
|
Respiratory, thoracic, and mediastinal disorders |
||
|
Dyspnea |
16 (13) |
19 (23) |
|
Pleural effusion |
12 (10) |
13 (16) |
aIncludes PT terms face swelling, conjunctival edema, eye edema, eyelid edema, orbital edema, periorbital edema, face edema, mouth edema, pharyngeal edema, peripheral edema, edema, generalized edema, localized edema, peripheral swelling, testicular edema.
bIncludes PT terms of abdominal pain, upper abdominal, lower abdominal pain, abdominal discomfort, abdominal tenderness
cIncludes PT terms memory impairment, cognitive disorder, confusional state, disturbance in attention, mental impairment, somnolence, encephalopathy, mental status changes, disorientation, and abnormal thinking.
dIncludes PT terms rash, rash maculo-papular, rash erythematous, rash macular, rash generalized, and rash papular
Adapted from FDA Review
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved AYVAKIT based on evidence from one clinical trial (NCT02508532) of 204 patients with GIST. The trial was conducted at 17 sites the United States, Europe and Asia.
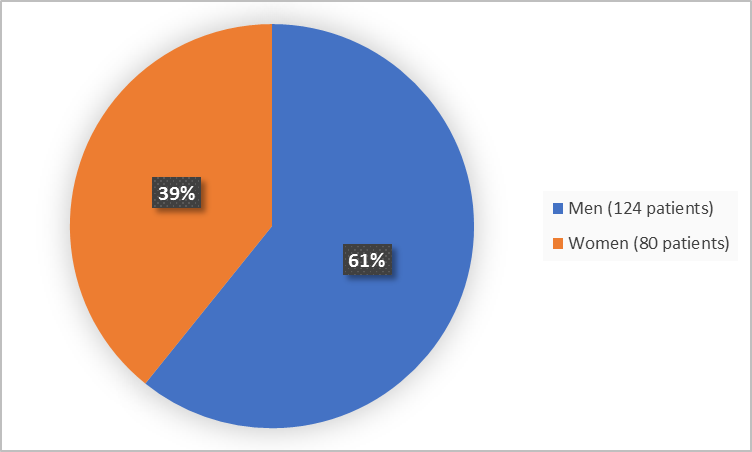
Figure 1 summarizes how many men and women were in the clinical trial.
Figure 1. Baseline Demographics by Sex (safety population)
FDA Review
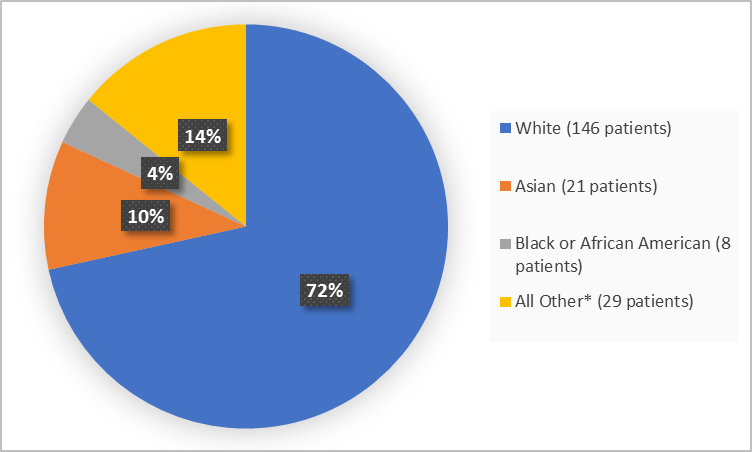
Figure 2 summarizes the percentage of patients by race in the clinical trial.
Figure 2. Baseline Demographics by Race (safety population)
*Includes American Indian or Alaska Native, Other and Unknown
FDA Review
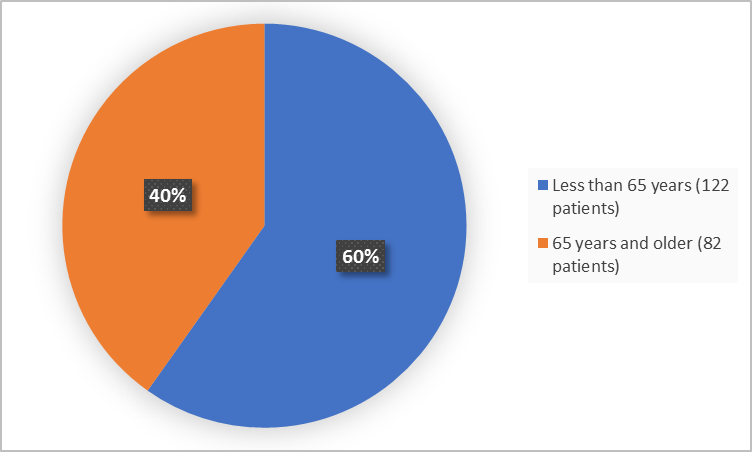
Figure 3 summarizes how many patients of certain age were in the clinical trial.
Figure 3. Baseline Demographics by Age (safety population)
FDA Review
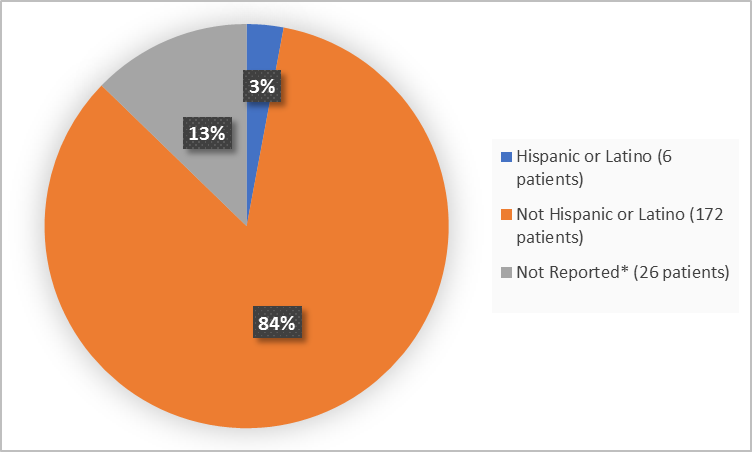
Figure 4. Baseline Demographics by Ethnicity (safety population)
*includes Not Reported and Unknown
FDA Review
Who participated in the trials?
The tables below summarize demographics of safety and efficacy populations, respectively.
Table 7. Demographics of Patients in the Clinical Trial 1-Safety Population
|
Demographic Parameter |
Safety Population |
|---|---|
|
Sex |
|
|
Men |
124 (61) |
|
Women |
80 (39) |
|
Race |
|
|
White |
146 (72) |
|
Asian |
21 (10) |
|
Black or African American |
8 (4) |
|
American Indian or Alaska Native |
2 (1) |
|
Other |
5 (3) |
|
Unknowna |
22 (11) |
|
Age |
|
|
Mean years (SD) |
59.5 (11.1) |
|
Median years (range) |
62 (29,90) |
|
Age Group |
|
|
<65 years |
122 (60) |
|
≥65 years |
82 (40) |
|
Ethnicity |
|
|
Not Hispanic or Latino |
172 (84) |
|
Hispanic/Latino |
6 (3) |
|
Not reporteda |
14 (7) |
|
Unknown |
12 (6) |
|
Region |
|
|
Europe |
95 (47) |
|
United States |
92 (45) |
|
Asia |
17 (8) |
a Data on race and/or ethnicity were not collected in France country because of local regulations.
FDA Review
Table 8. Demographics of Patients in the Clinical Trial 1-Efficacy Population
|
Demographic Parameter |
AYVAKIT |
|---|---|
|
Sex |
|
|
Men |
29 (67) |
|
Women |
14 (33) |
|
Race |
|
|
White |
29 (67) |
|
Asian |
6 (14) |
|
Black or African American |
3 (7) |
|
Other |
1 (2) |
|
Unknowna |
4 (9) |
|
Age |
|
|
Mean (SD) |
61.9 (11.8) |
|
Median |
64 |
|
Min, Max |
29,90 |
|
Age Group |
|
|
< 65 years |
24 (56) |
|
≥ 65 years |
19 (44) |
|
Ethnicity |
|
|
Not Hispanic or Latino |
37 (86) |
|
Hispanic or Latino |
1 (2) |
|
Not reporteda |
4 (1) |
|
Region |
|
|
Europe |
25 (58) |
|
US |
13 (30) |
|
Asia |
5 (12) |
a Data on race and/or ethnicity were not collected in France country because of local regulations.
FDA Review
How were the trials designed?
There was one trial that evaluated the benefits and side effects of AYVAKIT. Patients had unresectable or metastatic GIST with mutations in PDGFRA exon 18. The most common mutation was the PDGFRA D842V mutation.
All patients received AYVAKIT once daily until disease progression or unacceptable toxicity.
The benefit of AYVAKIT was evaluated by measuring the percentage of patients who experienced partial or complete tumor shrinkage and the duration of shrinkage.
How were the trials designed?
The safety and efficacy of AYVAKIT for the treatment of GIST were evaluated in one trial.
This was a multicenter, open-label, single-arm trial in adult patients with unresectable or metastatic GIST, harboring a PDGFRA exon 18 mutation. AYVAKIT was administered orally once daily (300 mg or 400 mg) until disease progression or unacceptable toxicity.
The primary endpoint was overall response rate (ORR), defined as the proportion of patients who achieved either a complete or partial response. ORR was based on disease assessment by independent radiological review using modified RECIST v1.1 criteria, in which lymph nodes and bone lesions were not target lesions and progressively growing new tumor nodules within a pre-existing tumor mass progressed. An additional efficacy outcome measure was duration of response (DOR).
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.