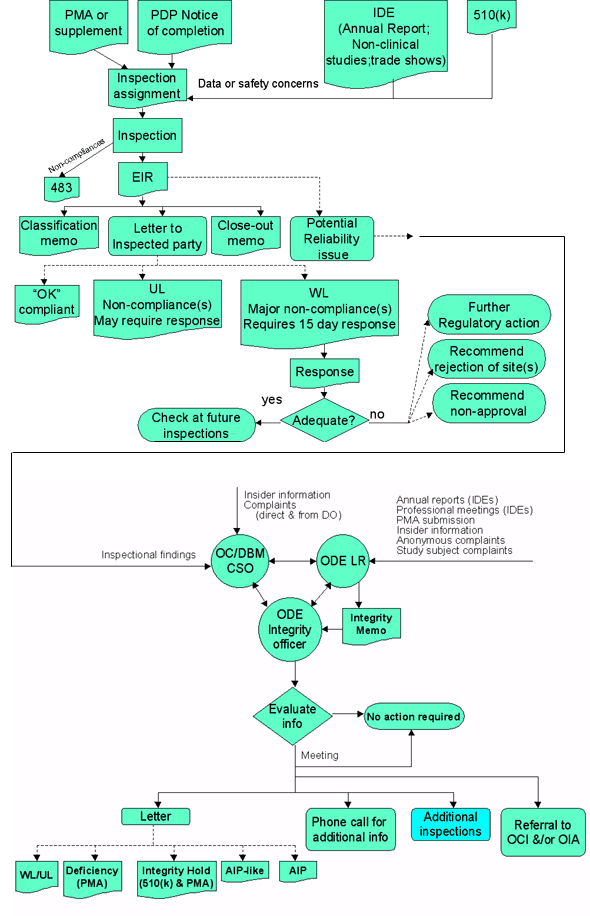
Summary Chart
KEY
483 - form FDA 483 - list of non-compliances left with inspected party
510(k) - submission for clearance as substantially equivalent
AIP - Application Integrity Policy
BIMO - Bioresearch Monitoring
CAP - Corrective Action Plan
CSO - Consumer Safety Officer
CST - Consumer Safety Technician
DBM - Division of Bioresearch Monitoring
DIB - Director of Investigations Branch
DO - District Office
EIR - Establishment Inspection Report
GMP - Good Manufacturing Practices
IDE - Investigational Device Exemption
LR - Lead reviewer
NAI - no action indicated
OAI - official action indicated
OC - Office of Compliance
OCI - Office of Criminal Investigation
ODE - Office of Device Evaluation
OIA - Office of Internal Affairs
PAP - Promotion and Advertising Policy
PMA - Premarket approval
PDP - Product development protocol
POS - Program Operations Staff
SOP - Standard Operating Procedure
UL - Untitled Letter
VAI - voluntary action indicated
WL - Warning Letter