Drug Trials Snapshots: OCALIVA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to OCALIVA Prescribing Information for complete information.
OCALIVA (obeticholic acid)
(oh’ kal i vah)
Intercept Pharmaceuticals
Approval date: May 27, 2016
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
OCALIVA is a drug for the treatment of primary biliary cholangitis (PBC) in adults. OCALIVA is to be used
- with another drug called ursodeoxycholic acid in patients whose liver enzyme tests do not adequately improve after using ursodeoxycholic acid for an appropriate period of time
or
- on its own when patients cannot take ursodeoxycholic acid because of side effects
Primary biliary cholangitis is an autoimmune disease that causes the small bile ducts in the liver to become inflamed, damaged and destroyed. This may lead to liver failure, cirrhosis and death.
How is this drug used?
OCALIVA is a tablet taken by mouth once a day. The daily dose may be increased after 3 months in order to achieve a greater improvement in liver enzyme tests, as long as the side effects are tolerated.
What are the benefits of this drug?
OCALIVA lowered the level of a liver enzyme that is found in the blood, and that is elevated in patients with PBC. It is believed that lowering this laboratory measurement may predict clinical benefit in patients. More trials are ongoing to confirm the clinical benefit.
What are the benefits of this drug (results of trials used to assess efficacy)?
The tables below summarize efficacy results after 12 months of therapy with OCALIVA based on the composite endpoint.
Table 2. Percentage of Adult Patients with PBC Achieving the Primary Composite Endpoint at Month 12 in Trial 1 by Treatment Arm with or without UDCAa
| OCALIVA 10 mg (N = 73) |
OCALIVA Titrationb (N = 70) |
Placebo (N = 73) |
|
|---|---|---|---|
| Primary Composite Endpointc | |||
| Responder rate, (%)d [95% CI] |
48 [36, 60] |
46 [34, 58] |
10 [4, 19] |
| Components of Primary Endpointe | |||
| ALP less than 1.67-times ULN, n (%) Decrease in ALP of at least 15%, n (%) Total bilirubin less than or equal to ULNf, n (%) |
40 (55) 57 (78) 60 (82) |
33 (47) 54 (77) 62 (89) |
12 (16) 21 (29) 57 (78) |
aIn the trial there were 16 patients (7%) who were intolerant and did not receive concomitant UDCA: 6 patients (8%) in the OCALIVA 10 mg arm, 5 patients (7%) in the OCALIVA titration arm, and 5 patients (7%) in the placebo arm.
bPatients randomized to OCALIVA titration received OCALIVA 5 mg for the initial 6 month period. At Month 6, patients who were tolerating OCALIVA, but had an ALP 1.67-times ULN or greater, and/or total bilirubin greater than ULN, or less than 15% ALP reduction were eligible for titration from 5 mg once daily to 10 mg once daily for the final 6 months of the trial.
cPercentage of patients achieving a response, defined as an ALP less than 1.67-times the ULN, total bilirubin less than or equal to the ULN, and an ALP decrease of at least 15%. Missing values were considered a non-response. The exact test was used to calculate the 95% CIs.
dp<0.0001 for OCALIVA titration and OCALIVA 10 mg arms versus placebo. P-values are obtained using the Cochran– Mantel–Haenszel General Association test stratified by intolerance to UDCA and pretreatment ALP greater than 3-times ULN and/or AST greater than 2-times ULN and/or total bilirubin greater than ULN.
eResponse rates were calculated based on the observed case analysis (i.e., [n=observed responder]/[N=ITT population]); percentage of patients with Month 12 values are 86%, 91% and 96% for the OCALIVA 10 mg, OCALIVA titration and placebo arms, respectively.
fThe mean baseline total bilirubin value was 0.65 mg/dL, and was less than or equal to the ULN in 92% of the enrolled patients.
OCALIVA Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
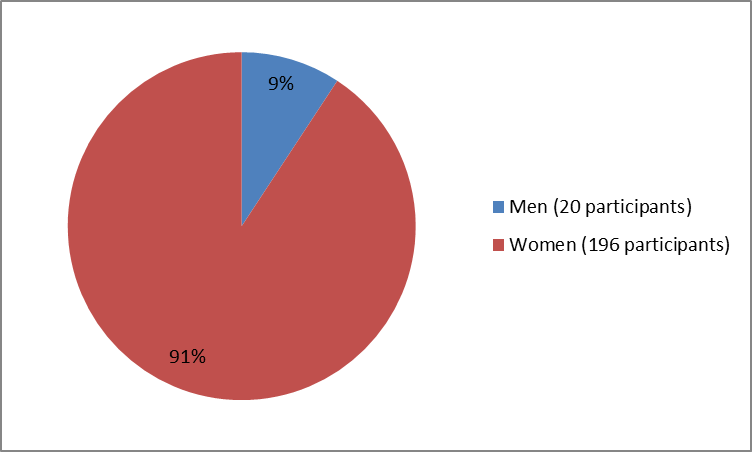
- Sex: The majority of participants in the clinical trials were women. Differences between sexes could not be determined due to the small number of male participants.
- Race: The majority of participants in the clinical trials were white. Differences among races could not be determined due to the small number of participants from other races.
- Age: OCALIVA worked similarly in participants younger and older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The table below summarizes efficacy results by subgroup.
Table 4. Efficacy Subgroup Analysis
| Demographic parameter | 10 mg OCA (N = 73) |
OCA Titration (N = 70) |
Placebo (N = 73) |
|---|---|---|---|
| Age groups | |||
| Age <> | n=56 | n=60 | n=60 |
| Response at Month 12 – n (%) | 30 (53.6) | 28 (46.7) | 7 (11.7) |
| Age ≥ 65 | n=17 | n=10 | n=13 |
| Response at Month 12 –n (%) | 5 (29.4) | 4 (40.0) | 0 |
| Sex | |||
| Men | n=10 | n=5 | n=5 |
| Response at Month 12 – n (%) | 3 (30.0) | 3 (60.0) | 0 |
| Women | n=63 | n=65 | n=68 |
| Response at Month 12 – n (%) | 32 (50.8) | 29 (44.6) | 7 (10.3) |
| Race | |||
| White | n=70 | n=67 | n=66 |
| Response at Month 12 – n (%) | 34 (48.6) | 31 (46.3) | 6 (9.1) |
| Non-White | n=3 | n=3 | n=7 |
| Response at Month 12 –n (%) | 1 (33.3) | 1 (33.3) | 1 (14.3) |
Adapted from FDA Statistical review
What are the possible side effects?
The most common side effects are itching of the skin, fatigue, abdominal pain and discomfort, joint pain, throat pain, dizziness, and constipation.
Treatment with OCALIVA may be associated with severe problems including liver injury, severe itching and lowering of “good cholesterol”.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions that occurred in ≥ 5% of patients in either OCALIVA treatment group and at an incidence ≥ 1% higher than placebo.
Table 5. Most Common Adverse Reactionsa in Adult Patients with PBC by Treatment Arm with or without UDCAa
| Adverse Reactionb | |||
|---|---|---|---|
| OCALIVA 10 mg N = 73 % |
OCALIVA Titrationc N = 70 % |
Placebo N = 73 % |
|
| Pruritusd | 70 | 56 | 38 |
| Fatiguee | 25 | 19 | 15 |
| Abdominal pain and discomfortf | 10 | 19 | 14 |
| Rashg | 10 | 7 | 8 |
| Arthralgia | 10 | 6 | 4 |
| Oropharyngeal pain | 8 | 7 | 1 |
| Dizzinessh | 7 | 7 | 5 |
| Constipation | 7 | 7 | 5 |
| Peripheral Edema | 7 | 3 | 3 |
| Palpitations | 7 | 3 | 1 |
| Pyrexia | 7 | 0 | 1 |
| Thyroid function abnormalityI | 4 | 6 | 3 |
| Eczema | 3 | 6 | 0 |
aIn the trial there were 16 patients (7%) who were intolerant and did not receive concomitant UDCA: 6 patients (8%) in the OCALIVA 10 mg arm, 5 patients (7%) in the OCALIVA titration arm, and 5 patients (7%) in the placebo arm.
bOccurring in greater than or equal to 5% of patients in either OCALIVA treatment arm and at an incidence greater than or equal to1% higher than in the placebo treatment arm.
cPatients randomized to OCALIVA titration received OCALIVA 5 mg once daily for the initial 6 month period. At Month 6, patients who were tolerating OCALIVA, but had an ALP 1.67-times ULN or greater, and/or total bilirubin greater than ULN, or less than 15% ALP reduction were eligible for titration from 5 mg once daily to 10 mg once daily for the final 6 months of the trial.
dIncludes skin eruptions, prurigo, pruritus, pruritus generalized, eye pruritus, ear pruritus, anal pruritus, vulvovaginal pruritus, and rash pruritic.
eIncludes fatigue, tiredness and asthenia.
fIncludes abdominal pain upper, abdominal pain, abdominal discomfort, abdominal pain lower, abdominal tenderness, and gastrointestinal pain.
gIncludes urticaria, rash, rash macular, rash papular, rash maculo-papular, heat rash, urticaria cholinergic.
hIncludes dizziness, syncope, presyncope.
iIncludes thyroxine free decreased, blood thyroid stimulating hormone increased, hypothyroidism.
Were there any differences in side effects among sex, race and age?
- Sex: The majority of participants in the clinical trials were women. Differences in side effects between sexes could not be determined due to the small number of male participants.
- Race: The majority of participants in the clinical trials were white. Differences in side effects among races could not be determined due to the small number of participants from other races.
- Age: The occurrence of common side effects was similar in participants younger and older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes pruritus by subgroup.
Table 6. Subgroup Analysis of Pruritus
| Demographic parameter | OCALIVA 10 mg (N = 73) |
OCALIVA Titration (N = 70) |
Placebo (N = 73) |
||||
|---|---|---|---|---|---|---|---|
| Subgroup n |
Subjects (%) |
Subgroup n |
Subjects (%) |
Subgroup n |
Subjects (%) |
||
| Age | |||||||
| <65> | 56 | 40 (71) | 60 | 35 (58) | 60 | 22 (37) | |
| ≥65 years | 17 | 10 (59) | 10 | 4 (40) | 13 | 6 (46) | |
| Sex | |||||||
| Women | 63 | 44 (70) | 65 | 37 (57) | 68 | 26 (38) | |
| Men | 10 | 6 (60) | 5 | 2 (40) | 5 | 2 (40) | |
| Race | |||||||
| White | 70 | 47 (67) | 67 | 37 (55) | 66 | 24 (36) | |
| Other | 3 | 3 (100) | 3 | 2 (67) | 7 | 4 (57) | |
Clinical trial data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved OCALIVA primarily based on evidence from one clinical trial of 216 patients with primary biliary cholangitis. The trial was conducted in North America, Europe and Australia.
The figure below summarizes how many men and women were in the clinical trial.
Figure 1. Baseline Demographics by Sex
Clinical trial data
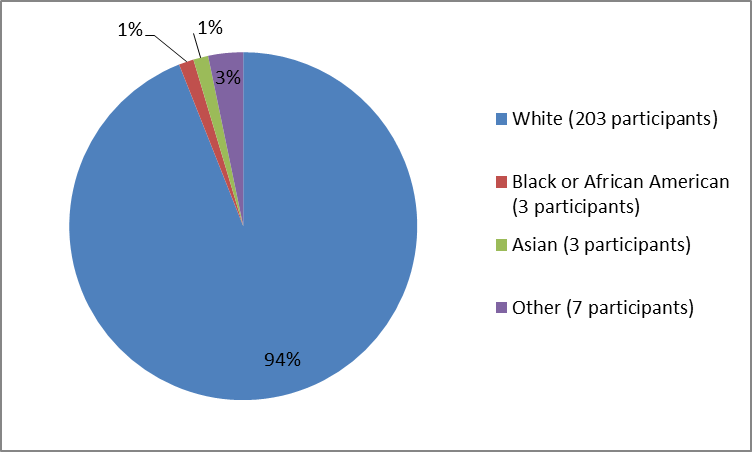
Figure 2 and Table 1 below summarize the percentage of participants by race in the clinical trial.
Figure 2. Baseline Demographics by Race
Clinical trial data
Table 1. Baseline Demographics by Race
| Race | Number of Participants | Percentage |
|---|---|---|
| White | 203 | 94% |
| Black or African American | 3 | 1% |
| Asian | 3 | 1% |
| Other | 7 | 3% |
Clinical trial data
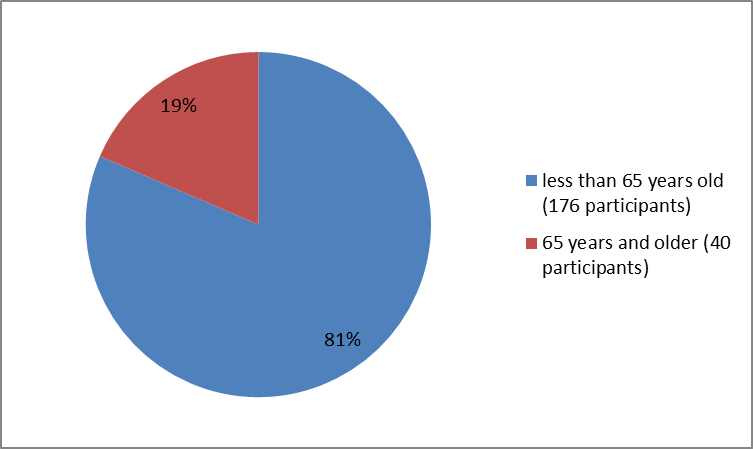
Figure 3 summarizes the percentage of participants by age group in the clinical trials.
Figure 3. Baseline Demographics by Age
Clinical trial data
Who participated in the trials?
The table below summarizes demographics of patients in the clinical trials.
Table 7. Baseline Demographics of Patients in the Clinical Trials (Safety Population)
| Number of Patients | OCALIVA 10 mg (N = 73) |
OCALIVA Titration (N = 70) |
Placebo (N = 73) |
Total (N = 216) |
|---|---|---|---|---|
| Age (years) | ||||
| N | 73 | 70 | 73 | 216 |
| Mean (SD) | 56.2 (11.0) | 55.8 (10.5) | 55.5 (10.0) | 55.8 (10.5) |
| Median | 56.0 | 54.5 | 55.0 | 55.0 |
| Min, Max | 30, 86 | 29, 83 | 35, 78 | 29, 86 |
| Age subgroups, n (%) | ||||
| <65> | 56 (77) | 60 (86) | 60 (82) | 176 (81) |
| >65 years | 17 (23) | 10 (14) | 13 (18) | 40 (19) |
| Sex, n (%) | ||||
| Men | 10 (14) | 5 (7) | 5 (7) | 20 (9) |
| Women | 63 (86) | 65 (93) | 68 (93) | 196 (91) |
| Race, n (%) | ||||
| White | 70 (96) | 67 (96) | 66 (90) | 203 (94) |
| Black or African American | 1(1) | 1 (1) | 1 (1) | 3 (1) |
| Asian | 1 (1) | 1 (1) | 1(1) | 3 (1) |
| Other | 1 (1) | 1 (1) | 5 (7) | 7 (3) |
| Region, n (%) | ||||
| Europe | 51 (70) | 45 (64) | 49 (67) | 145 (67) |
| North America | 21 (29) | 20 (29) | 21 (29) | 62 (29) |
| Australia | 1 (1) | 5 (7) | 3 (4) | 9 (4) |
Adapted from Clinical review
How were the trials designed?
There was one trial that evaluated the benefit and side effects of OCALIVA. Patients were randomly assigned to receive either OCALIVA or placebo once daily for 12 months. Neither the patients nor the health care providers knew which treatment was being given until after the trials were completed.
The benefit of OCALIVA was evaluated by measuring the proportion of patients with improved liver enzymes after 12 months of treatment.
How were the trials designed?
The efficacy of OCALIVA was evaluated in a single 12-month, randomized, double-blind, placebo controlled, parallel-group trial. Enrolled participants had a diagnosis of primary biliary cholangitis (PBC), and alkaline phosphatase (ALP) 1.67-times upper limit of normal (ULN) or greater and/or total bilirubin was greater than 1-times ULN but less than 2-times ULN.
Patients were randomized (1:1:1) to receive either OCALIVA 10 mg once daily for the entire 12 months of the trial, (n=73); OCALIVA titration (5 mg once daily for the initial 6 months, with the option to increase to 10 mg once daily for the last 6 months, if the patient was tolerating OCALIVA but had ALP 1.67-times ULN or greater, and/or total bilirubin above ULN, or less than 15% ALP reduction) (n=70); or placebo (n=73).
Primary efficacy was a responder analysis evaluated at Month 12, where response was defined as a composite of three criteria: ALP less than 1.67-times the ULN, total bilirubin less than or equal to ULN, and an ALP decrease of at least 15%.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION