Drug Trials Snapshots: GENVOYA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the GENVOYA Prescribing Information for complete information.
GENVOYA (elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide) tablets
jen-VOY-uh
Gilead Sciences
Approval date: November 5, 2015
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
GENVOYA is a drug for the treatment of HIV-1 infection in adults and children 12 years of age and older. It is a fixed-dose combination tablet, which means GENVOYA includes more than one drug combined in a single tablet.
How is this drug used?
GENVOYA is a tablet that is taken by mouth with food once a day.
GENVOYA should not be given with other antiretroviral products and may have drug interactions with a number of other commonly used medications.
What are the benefits of this drug?
GENVOYA reduced viral load and is comparable to other treatments.
What are the benefits of this drug results of trials used to assess efficacy)?
Efficacy information for GENVOYA can be found in PRESCRIBING INFORMATION.
The table below summarizes the primary efficacy endpoint (pooled virologic outcomes) at Week 48 for the pooled two clinical trials of treatment-naïve subjects.
Table 3. Pooled Virologic Outcomes of Randomized Treatment at Week 48 for the Pooled Trials of Treatment-Naïve Subjects
| GENVOYA (N=866) |
STRIBILD (N=867) |
|
|---|---|---|
| HIV-1 RNA < 50=""> | 92% | 90% |
| Treatment Difference | 2.0% (95% CI: -0.7% to 4.7%) | |
| HIV-1 RNA ≥ 50 copies/mLb | 4% | 4% |
| No Virologic Data at Week 48 Window | 4% | 6% |
| Discontinued Study Drug Due to AE or Deathc | 1% | 2% |
| Discontinued Study Drug Due to Other Reasons and Last Available HIV-1 RNA < 50 copies>d | 2% | 4% |
| Missing Data During Window but on Study Drug | 1% | <1% |
a. Week 48 window was between Day 294 and 377 (inclusive).
b. Included subjects who had ≥ 50 copies/mL in the Week 48 window; subjects who discontinued early due to lack or loss of efficacy; subjects who discontinued for reasons other than an adverse event (AE), death or lack or loss of efficacy and at the time of discontinuation had a viral value of ≥ 50 copies/mL.
c. Includes subjects who discontinued due to AE or death at any time point from Day 1 through the time window if this resulted in no virologic data on treatment during the specified window.
d. Includes subjects who discontinued for reasons other than an AE, death or lack or loss of efficacy; e.g., withdrew consent, loss to follow-up, etc.
GENVOYA Prescribing Information, Table 13
The table below summarizes the primary endpoint in the trial of virologically-suppressed subjects who switched to GENVOYA.
Table 4. Virologic Outcomes at Week8 in Virologically-Suppressed Subjects who Switched to GENVOYA
| GENVOYA (N=799) |
ATRIPLA or TRUVADA+atazanavir+cobicistat or ritonavir or STRIBILD (N=397) |
|
|---|---|---|
| HIV-1 RNA < 50 copies/mL | 96% | 93% |
| HIV-1 RNA ≥ 50 copies/mLb | 1% | 1% |
| No Virologic Data at Week 48 Window | 3% | 6% |
| Discontinued Study Drug Due to AE or Deathc | 1% | 1% |
| Discontinued Study Drug Due to Other Reasons and Last Available HIV-1 RNA < 50 copies>d | 1% | 4% |
| Missing Data During Window but on Study Drug | 2% | 1% |
GENVOYA Prescribing Information, Table 14
Refer to PRESCRIBING INFORMATION for efficacy data on HIV-1 infected subjects with renal impairment and HIV-1 treatment-naïve adolescent subjects aged 12 to less than 18.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
Subgroup analyses were conducted for sex, race and age.
- Sex: GENVOYA worked similarly in men and women.
- Race: GENVOYA worked similarly in Whites, Blacks, and Asians.
- Age: The majority of patients in the trials were below 65 years of age. Differences in how well GENVOYA worked between those below and above 65 years of age could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Subgroup analysis was conducted for the clinical trials that supported approval. Below are the separate analyses for the primary endpoint in two large clinical trials in treatment-naïve subjects (Trails 104 and 111), the trial in virologically-suppressed adults, and the trial in virologically-suppressed adults with renal impairment. The trial of adolescents had too few subjects to perform adequate subgroup analysis.
Table 5. Virologic Outcome by Subgroup for the Clinical Trial of Treatment-Naïve Patients (Trial 104)
| Subgroup | GENVOYA | STRIBILD |
|---|---|---|
| Sex Women Men |
67/71 (94.4%) 339/364 (93.1%) |
52/56 (92.9%) 347/376 (92.3%) |
| Race White Black Asian Other |
235/250 (94%) 82/94 (87.2%) 79/81 (97.5%) 10/10 (100%) |
243/255 (95.3%) 64/81 (79%) 81/85 (95.3%) 11/11 (100%) |
| Age <65 ≥65 |
404/433 (93.3%) 2/200 (100%) |
393/426 (92.9%) 6/6 (100%) |
FDA Statistical Review
Table 6. Virologic Outcome by Subgroup for the Clinical Trial of Treatment-Naïve Patients (Trial 111)
| Subgroup | GENVOYA | STRIBILD |
|---|---|---|
| Sex Women Men |
59/62 (95.2%) 331/369 (89.7%) |
59/71 (83.1%) 324/364 (89%) |
| Race White Black Asian Other |
214/235 (91.1%) 115/129 (89.1%) 17/20 (85%) 44/47 (93.6%) |
217/243 (89.3%) 113/132 (85.6%) 13/16 (81.3%) 40/44 (90.9%) |
| Age <65 ≥65 |
389/430 (90.5%) 1/1 (100%) |
379/431 (87.9%) 4/4 (100%) |
FDA Statistical Review
Table 7. Virologic Outcome by Subgroup for the Clinical Trial of Virologically- Suppressed adults (Trial 109)
| Subgroup | GENVOYA | Control |
|---|---|---|
| Sex Women Men |
73/78 (93.6%) 687/721 (95.3%) |
35/37 (94.6%) 339/360 (94.2%) |
| Race White Black Asian Other |
511/537 (95.2%) 149/158 (94.3%) 47/49 (95.9%) 53/55 (96.4%) |
247/261 (94.6%) 87/94 (92.6%) 24/26 (92.3%) 16/16 (100%) |
| Age <65 ≥65 |
750/789 (95.1%) 10/10 (100%) |
369/392 (94.1%) 5/5 (100%) |
FDA Statistical Review
Table 8. Virologic Outcome by Subgroup for the Clinical Trial in Virologically- suppressed adults with renal impairment (Trial 112)
| Subgroup | GENVOYA | STRIBILD |
|---|---|---|
| Sex Women Men |
4/4 (100%) 95/108 (88%) |
1/1 (100%) 48/57 (84.2%) |
| Race White Black Asian |
68/74 (91.9%) 28/35 (80%) 3/3 (100%) |
36/40 (90%) 12/16 (75%) 1/2 (50%) |
| Age <65 ≥65 |
98/111 (88.3%) 1/1 (100%) |
49/58 (84.5%) 0 |
FDA Statistical Review
What are the possible side effects?
The most common side effect associated with GENVOYA is nausea. Serious side effects include new or worsening kidney problems, decreased bone mineral density, fat redistribution and changes in the immune system (immune reconstitution syndrome).
GENVOYA can cause a buildup of lactic acid in the blood and severe liver problems, both of which can be fatal.
Patients receiving GENVOYA experienced significantly higher increases in serum cholesterol (both total and LDL, or “bad”, cholesterol) compared to patients receiving another HIV medication in the clinical trials.
GENVOYA is not approved to treat chronic hepatitis B virus infection.
What are the possible side effects (results of trials used to assess safety)?
The primary safety assessment of GENVOYA in treatment-naïve adults was based on Week 48 pooled data from 1733 subjects in two randomized, double-blind, active-controlled trials (called Study 104 and Study 111) in antiretroviral treatment-naïve HIV-1 infected adult subjects. The table below summarizes adverse reactions from these 2 trials.
Table 9. Adverse Reactionsa (All Grades) Reported in ≥ 5% of HIV-1 Infected Treatment Naïve Adults Receiving GENVOYA in Studies 104 and 111 (Week 48 analysis)
| GENVOYA N=866 |
STRIBILD N=867 |
|
|---|---|---|
| GASTROINTESTINAL DISORDERS | ||
| Diarrhea | 7% | 9% |
| Nausea | 10% | 13% |
| GENERAL DISORDERS AND ADMINISTRATION SITE CONDITIONS | ||
| Fatigue | 5% | 4% |
| NERVOUS SYSTEM DISORDERS | ||
| Headache | 6% | 5% |
a. Frequencies of adverse reactions are based on all adverse events attributed to study drugs by the investigator.
GENVOYA Prescribing Information, Table 2
In the clinical trial of virologically suppressed adults, the adverse reaction profile of GENVOYA was similar to that of treatment-naïve subjects.
In the 24-week clinical trial of patients with renal impairment (baseline eGFR 30 to 69 mL per minute) who received GENVOYA, mean serum creatinine was 1.5 mg per dL at both baseline and Week 24. Median UPCR was 161 mg per gram at baseline and 93 mg per gram at Week 24. In the clinical trial of pediatric patients, the adverse reaction profile was similar to that in the adults trials.
Additional safety information is described in Section 6 of GENVOYA Prescribing Information.
Were there any differences in side effects among sex, race and age?
Subgroup analyses were conducted for sex, race and age.
- Sex: The risk of side effects was similar in men and women.
- Race: The risk of side effects was similar in black and non-black patients.
- Age: The majority of patients in the trials were below 65 years of age. Differences in the risk of side effects between those below and above 65 years of age could not be determined.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
According to the primary review:
- the overall incidence of treatment-emergent adverse events in the pivotal trials was similar in men and women.
- the incidence of adverse events in the pivotal trials was similar in black and non-black patients.
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved GENVOYA based on evidence from 4 clinical trials of 3,171 adults and one trial of 23 children with HIV.
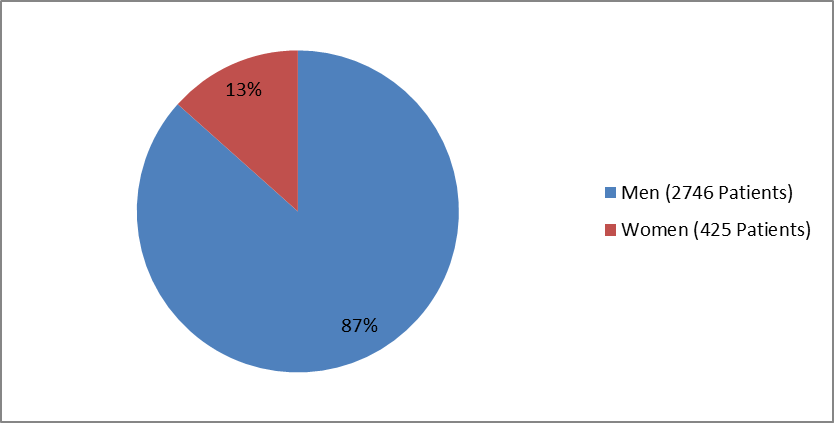
Figure 1 summarizes how many men and women were enrolled in the clinical trials in adults.
Figure 1. Baseline Demographics by Sex--Adults
Clinical Trial Data
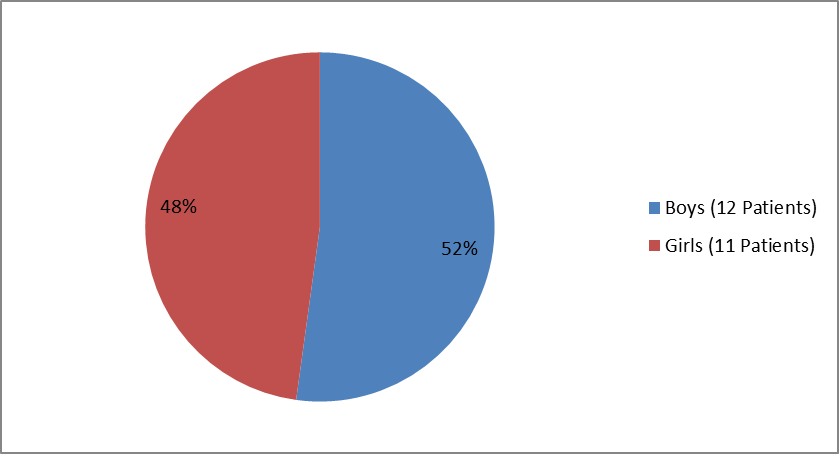
Figure 2 summarizes how many boys and girls were enrolled in the clinical trial in children.
Figure 2. Baseline Demographics by Sex for Children
Clinical Trial Data
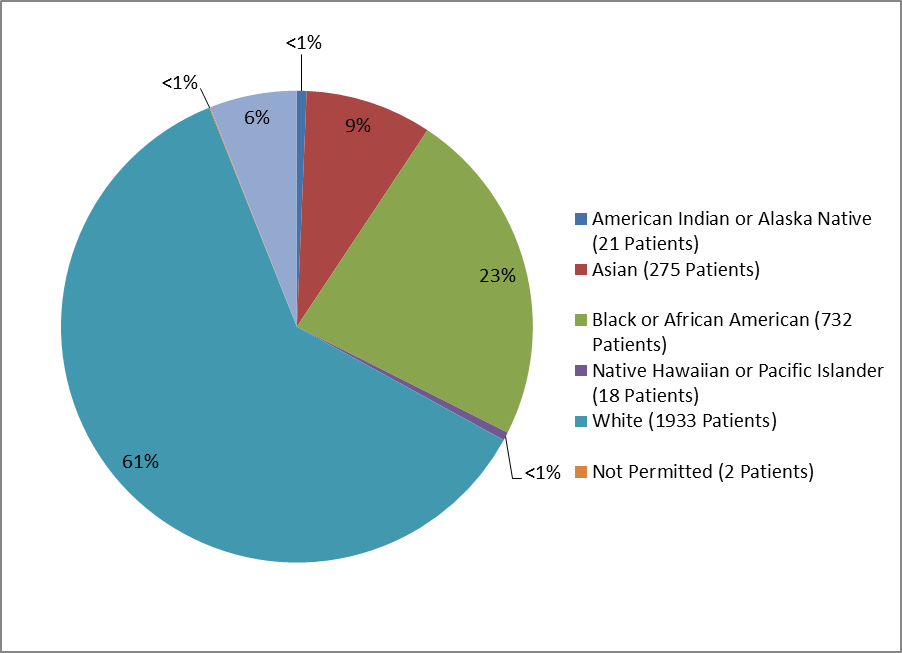
Figure 3 and Table 1 summarize the percentage of adults by race enrolled in the clinical trials.
Figure 3. Baseline Demographics by Race--Adults
<1%=less than="">
Not permitted=collection of race data not permitted in all countries
Clinical Trial Data
Table 1. Demographics of Efficacy Trials by Race--Adults
| Race | Number of Patients | Percentage |
|---|---|---|
| American Indian or Alaska Native | 21 | <1% |
| Asian | 275 | 9% |
| Black or African American | 732 | 23% |
| Native Hawaiian or Pacific Islander | 18 | <1% |
| White | 1933 | 61% |
| Not Permitted | 2 | <1% |
<1%=less than="">
Not permitted=collection of race data not permitted in all countries
Clinical Trial Data
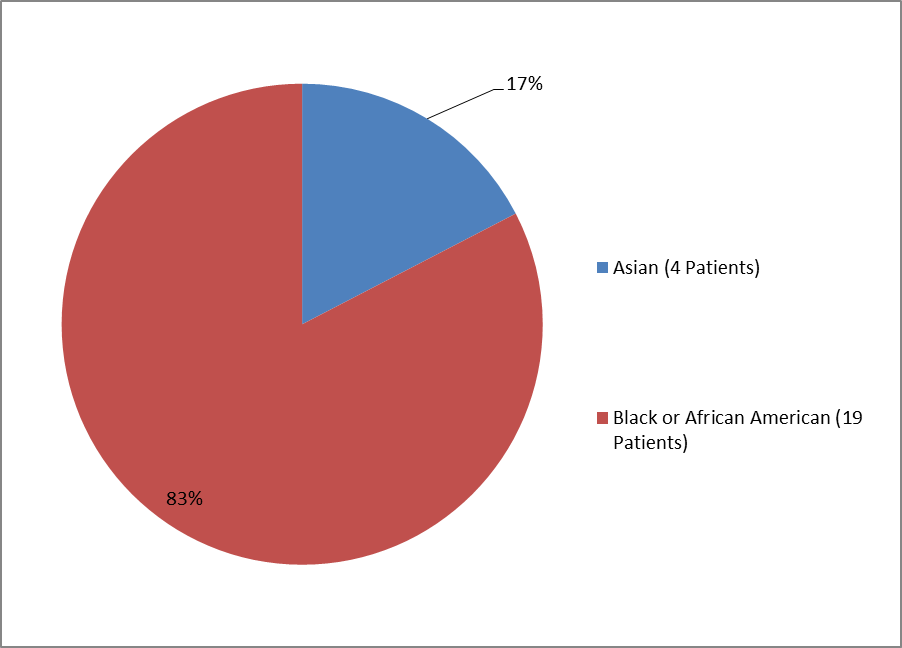
Figure 4 and Table 2 summarize the percentage of children by race enrolled in the clinical trial.
Figure 4. Baseline Demographics by Race—Children
Clinical Trial Data
Table 2. Baseline Demographics by Race—Children
| Race | Number of Patients | Percentage |
|---|---|---|
| Asian | 4 | 17% |
| Black or African American | 19 | 83% |
Clinical Trial Data
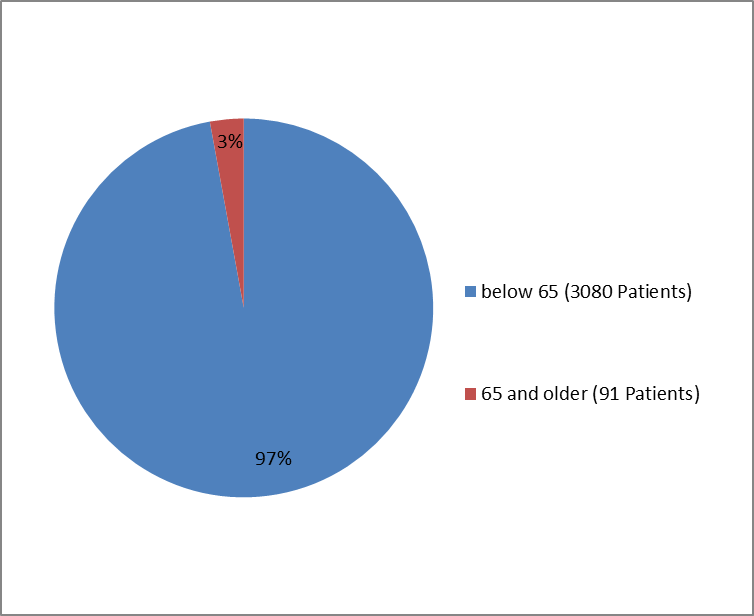
Figure 5 summarizes how many patients adults by age were enrolled in the clinical trials.
Figure 5. Baseline Demographics by Age—Adults
Clinical Trial Data
Who participated in the trials?
The tables below summarize baseline demographics for the pooled adult clinical trials (Table 10) and for the clinical trial in children (Table 11).
Table 10. Baseline Demographics for the 4 Pooled Adult Trials (104, 111, 109, and 112)
| GENVOYA | Comparator | Total | |
|---|---|---|---|
| (N=1907) | (N=1264) | (N=3171) | |
| Sex | |||
| Male | 1646 (86.3%) | 1100 (87.0%) | 2746 (86.6%) |
| Female | 261 (13.7%) | 164 (13.0%) | 425 (13.4%) |
| Age Group | |||
| 18 - 64 years | 1831 (96.0%) | 1249 (98.8%) | 3080 (97.1%) |
| >= 65 years | 76 (4.0%) | 15 (1.2%) | 91 (2.9%) |
| Race | |||
| American Indian or Alaska Native | 11 (0.6%) | 10 (0.8%) | 21 (0.7%) |
| Asian | 163 (8.5%) | 112 (8.9%) | 275 (8.7%) |
| Black | 425 (22.3%) | 307 (24.3%) | 732 (23.1%) |
| Native Hawaiian or Pacific Islander | 13 (0.7%) | 5 (0.4%) | 18 (0.6%) |
| White | 1174 (61.6%) | 759 (60.0%) | 1933 (61.0%) |
| Not Permitted | 2 (0.1%) | 0 | 2 (<0.1% |
| Other | 119 (6.2%) | 71 (5.6%) | 190 (6.0%) |
| Ethnicity | |||
| Hispanic or Latino | 404 (21.2%) | 233 (18.4%) | 637 (20.1%) |
| Not Hispanic or Latino | 1500 (78.7%) | 1027 (81.3%) | 2527 (79.7%) |
| Not Permitted | 3 (0.2%) | 3 (0.2%) | 6 (0.2%) |
| Missing | 0 | 1 | 1 |
Clinical Trial Data
Table 11. Baseline Demographics for the Trial in Children
| All patients | |
|---|---|
| (N=23) | |
| Sex | |
| Male | 12 (52.2%) |
| Female | 11 (47.8%) |
| Race | |
| Asian | 4 (17.4%) |
| Black | 19 (82.6%) |
| Ethnicity | |
| Not Hispanic or Latino | 23 (100.0%) |
Clinical Trial Data
How were the trials designed?
GENVOYA was evaluated in four clinical trials in adults and one clinical trial in adolescents. In the adult trials, patients were randomly assigned to receive GENVOYA or another FDA approved HIV treatment. In the adolescent trial, all patients received GENVOYA.
How were the trials designed?
(no further info included).
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.